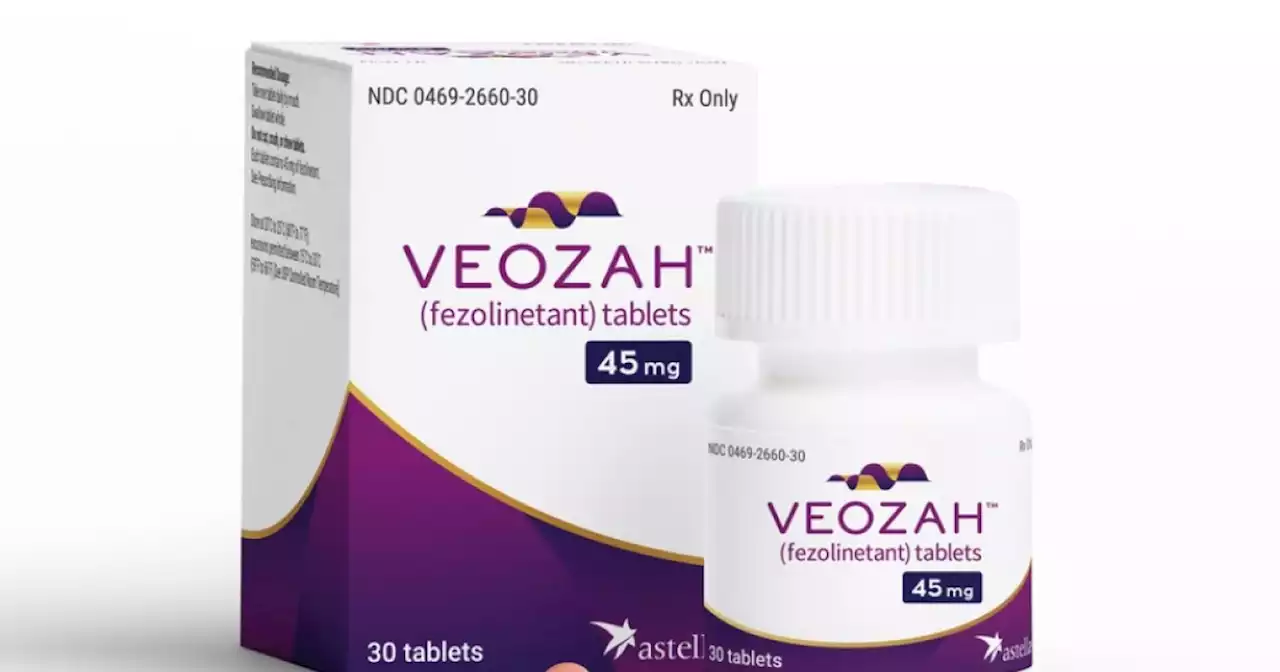
The FDA has approved a once-a-day pill that neurologically targets the body's temperature control center, which can calm menopausal hot flashes.
When a woman gets older, her period will eventually stop — a natural menopausal transition that can leave many women struggling with symptoms for years.
On Friday, the U.S. Food and Drug Administration approved a new type of drug to treat moderate to severe hot flashes caused by menopause. "Hot flashes as a result of menopause can be a serious physical burden on women and impact their quality of life. The introduction of a new molecule to treat moderate to severe menopausal hot flashes will provide an additional safe and effective treatment option for women," said Dr. Janet Maynard, director of the FDA’s Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine in the Center for Drug Evaluation and Research.
Menopause typically occurs between the ages of 45 and 55. It's marked by the body slowly producing less estrogen and progesterone — a transition that usually lasts about seven years. The reduction of hormones during menopause can trigger harsh symptoms, like hot flashes.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Japan's Otsuka Pharma gets FDA approval for Alzheimer's agitation drugThe U.S. Food and Drug Administration approved Otsuka Pharmaceutical's brexpiprazole to treat agitation in patients with Alzheimer's on Thursday, making it the first approved drug for the indication, the company said in a statement.
Japan's Otsuka Pharma gets FDA approval for Alzheimer's agitation drugThe U.S. Food and Drug Administration approved Otsuka Pharmaceutical's brexpiprazole to treat agitation in patients with Alzheimer's on Thursday, making it the first approved drug for the indication, the company said in a statement.
Lire la suite »
 FDA advisers endorse over-the-counter sales of birth control pillIn the U.S., contraceptive pills require a prescription, unlike in over 100 countries where oral contraceptives are available over-the-counter.
FDA advisers endorse over-the-counter sales of birth control pillIn the U.S., contraceptive pills require a prescription, unlike in over 100 countries where oral contraceptives are available over-the-counter.
Lire la suite »
 FDA advisers vote in favor of over-the-counter birth control pillAdvisers for the US Food and Drug Administration voted unanimously on Wednesday in support of making the birth-control pill Opill available over-the-counter, saying the benefits outweigh the risks.
FDA advisers vote in favor of over-the-counter birth control pillAdvisers for the US Food and Drug Administration voted unanimously on Wednesday in support of making the birth-control pill Opill available over-the-counter, saying the benefits outweigh the risks.
Lire la suite »
 Advisers to FDA Endorse Over-the-Counter Birth ControlOutside advisers to the U.S. Food and Drug Administration (FDA) voted 17-0 on Wednesday to endorse over-the-counter birth control pills.
Advisers to FDA Endorse Over-the-Counter Birth ControlOutside advisers to the U.S. Food and Drug Administration (FDA) voted 17-0 on Wednesday to endorse over-the-counter birth control pills.
Lire la suite »
 FDA advisers unanimously back over-the-counter birth control pillPanellists say that making the contraceptive available in the US without a prescription will benefit women’s health.
FDA advisers unanimously back over-the-counter birth control pillPanellists say that making the contraceptive available in the US without a prescription will benefit women’s health.
Lire la suite »
 FDA finalizes blood-donation rules to allow more gay, bisexual menThe updated FDA guidelines do away with a requirement that men who have sex with men abstain from sex for three months prior to giving blood.
FDA finalizes blood-donation rules to allow more gay, bisexual menThe updated FDA guidelines do away with a requirement that men who have sex with men abstain from sex for three months prior to giving blood.
Lire la suite »