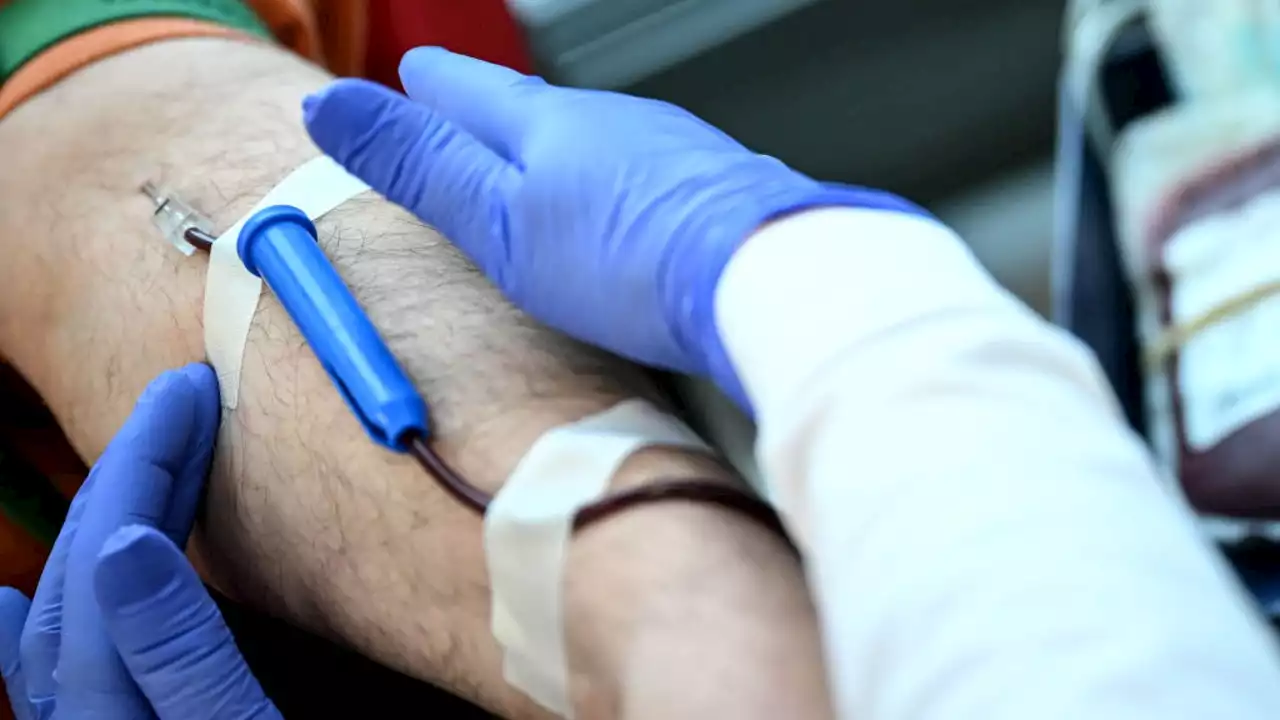
The updated FDA guidelines do away with a requirement that men who have sex with men abstain from sex for three months prior to giving blood.
Gay and bisexual
The Food and Drug Administration guidelines ease decades-old restrictions designed to protect the blood supply from HIV. The agencydo away with a requirement that men who have sex with men abstain from sex for three months prior to giving blood. The FDA said the new policy reflects the latest scientific evidence and is in line with rules in the U.K. and Canada."The implementation of these recommendations will represent a significant milestone for the agency and the LGBTQI+ community," Dr. Peter Marks, director of the FDA's center for biological therapies, said in a statement.Every June, the LGBTQ+ community and allies celebrate what has become "Pride Month.
Anyone who has ever tested positive for HIV will continue to be ineligible to donate blood. Those taking pills to prevent HIV through sexual contact will also still be barred, until three months after their last dose. The FDA noted that the medications, known as PrEP, can delay the detection of the virus in screening tests.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA Experts to Consider First Over-the-Counter Birth Control PillAs a U.S. Food Drug Administration advisory panel prepares to weigh whether to recommend that a birth control pill be sold over the counter in this country, a coalition of advocates on Monday called attention to the safety and effectiveness of the medication.
FDA Experts to Consider First Over-the-Counter Birth Control PillAs a U.S. Food Drug Administration advisory panel prepares to weigh whether to recommend that a birth control pill be sold over the counter in this country, a coalition of advocates on Monday called attention to the safety and effectiveness of the medication.
Lire la suite »
 FDA may allow birth control pill without prescription this summer as experts weigh dataHRA Pharma asked the Food and Drug Administration to allow over-the-counter sales of Opill after the Supreme Court said there was no federal right to abortion.
FDA may allow birth control pill without prescription this summer as experts weigh dataHRA Pharma asked the Food and Drug Administration to allow over-the-counter sales of Opill after the Supreme Court said there was no federal right to abortion.
Lire la suite »
 FDA Expands Use of Dapagliflozin to Broader Range of HFThe SGLT2 inhibitor is now indicated for treatment of heart failure across the full spectrum of left-ventricular ejection fraction ― including HF with mildly reduced and preserved ejection fraction.
FDA Expands Use of Dapagliflozin to Broader Range of HFThe SGLT2 inhibitor is now indicated for treatment of heart failure across the full spectrum of left-ventricular ejection fraction ― including HF with mildly reduced and preserved ejection fraction.
Lire la suite »
 FDA approves ALS treatment for patients with genetic diseaseThe FDA has fast-tracked a new ALS treatment for patients whose disease is genetic. Patients who have been getting the injections report a slowing of their disease progression and even renewed strength.
FDA approves ALS treatment for patients with genetic diseaseThe FDA has fast-tracked a new ALS treatment for patients whose disease is genetic. Patients who have been getting the injections report a slowing of their disease progression and even renewed strength.
Lire la suite »
 ‘Caught between a rock and a hard place’: FDA considers over-the-counter birth controlThe agency is slated to make a decision on approval of the daily hormonal pill by this summer.
‘Caught between a rock and a hard place’: FDA considers over-the-counter birth controlThe agency is slated to make a decision on approval of the daily hormonal pill by this summer.
Lire la suite »
 FDA debates health risks of birth control without prescriptionThe FDA may approve birth control pills over-the-counter by the summer, but expressed hesitation due to concerns over women misreading labels on the drug's health concerns.
FDA debates health risks of birth control without prescriptionThe FDA may approve birth control pills over-the-counter by the summer, but expressed hesitation due to concerns over women misreading labels on the drug's health concerns.
Lire la suite »