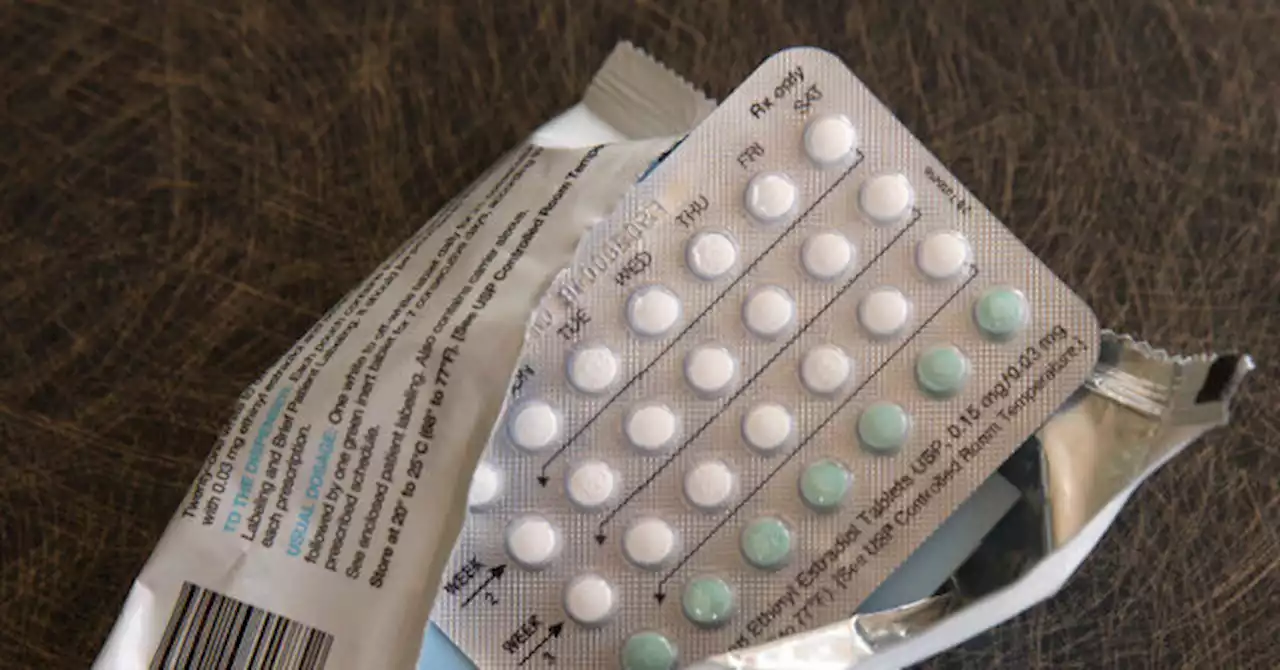
Outside advisers to the U.S. Food and Drug Administration (FDA) voted 17-0 on Wednesday to endorse over-the-counter birth control pills.
“Do I think we got perfect data? No. Do I think it was a perfect study? No. Do I think it was adequate to feel reassured that a large number of people can use this drug as intended? Yes,” said Cynthia Baur, a health literacy researcher at the University of Maryland who voted in favor of OTC access.
The abortion giant Planned Parenthood celebrated the news, calling the move a “huge step to increase access to birth control, especially for people who have the hardest time getting a prescription.” However, Students for Life of America President Kristan Hawkins released a statement via email calling the move“Making it easy for abusers to cover up their sexual abuse and statutory rape crimes with Online, No Test Chemical Abortion Pills or over-the-counter birth control sales is negligent public policy,” Hawkins said in part prior to Wednesday’s decision.
“It’s astounding that an agency established to keep women safe when using any substances is more interested in a quick sale than patient safety. President Biden’s FDA must stop prioritizing the goals of the abortion lobby and put women and young girls first,” she continued. “As I travel on campuses across the country, I find that young women – both pro-life and pro-choice – agree that hormonal birth control causes women many terrible problems. Outside of Washington D.C.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Gardopia Gardens food and nature conference to focus on food insecurity and climate changeThe 3rd Annual Food & Nature Conference will highlight what actions some local organizations are taking and offer simple solutions through onsite workshops
Gardopia Gardens food and nature conference to focus on food insecurity and climate changeThe 3rd Annual Food & Nature Conference will highlight what actions some local organizations are taking and offer simple solutions through onsite workshops
Lire la suite »
 FDA panel backs over-the-counter birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription. The positive vote on Wednesday by a Food and Drug Administration panel paves the way for what could be the first birth control pill available over the counter. The recommendation is not binding and the FDA is expected to make its decision on the drug this summer. Currently all contraceptive pills in the U.S. require a prescription. Dozens of medical and advocacy groups support making the pill available without a prescription to increase birth control options for women.
FDA panel backs over-the-counter birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription. The positive vote on Wednesday by a Food and Drug Administration panel paves the way for what could be the first birth control pill available over the counter. The recommendation is not binding and the FDA is expected to make its decision on the drug this summer. Currently all contraceptive pills in the U.S. require a prescription. Dozens of medical and advocacy groups support making the pill available without a prescription to increase birth control options for women.
Lire la suite »
 FDA advisory committees meeting to discuss over-the-counter birth controlAdvisory committees of the U.S. Food and Drug Administration are meeting Tuesday and Wednesday to review the first-ever application for an over-the-counter birth control pill.
FDA advisory committees meeting to discuss over-the-counter birth controlAdvisory committees of the U.S. Food and Drug Administration are meeting Tuesday and Wednesday to review the first-ever application for an over-the-counter birth control pill.
Lire la suite »
 FDA may allow birth control pill without prescription this summer as experts weigh dataHRA Pharma asked the Food and Drug Administration to allow over-the-counter sales of Opill after the Supreme Court said there was no federal right to abortion.
FDA may allow birth control pill without prescription this summer as experts weigh dataHRA Pharma asked the Food and Drug Administration to allow over-the-counter sales of Opill after the Supreme Court said there was no federal right to abortion.
Lire la suite »
 FDA Experts to Consider First Over-the-Counter Birth Control PillAs a U.S. Food Drug Administration advisory panel prepares to weigh whether to recommend that a birth control pill be sold over the counter in this country, a coalition of advocates on Monday called attention to the safety and effectiveness of the medication.
FDA Experts to Consider First Over-the-Counter Birth Control PillAs a U.S. Food Drug Administration advisory panel prepares to weigh whether to recommend that a birth control pill be sold over the counter in this country, a coalition of advocates on Monday called attention to the safety and effectiveness of the medication.
Lire la suite »
 FDA Expands Use of Dapagliflozin to Broader Range of HFThe SGLT2 inhibitor is now indicated for treatment of heart failure across the full spectrum of left-ventricular ejection fraction ― including HF with mildly reduced and preserved ejection fraction.
FDA Expands Use of Dapagliflozin to Broader Range of HFThe SGLT2 inhibitor is now indicated for treatment of heart failure across the full spectrum of left-ventricular ejection fraction ― including HF with mildly reduced and preserved ejection fraction.
Lire la suite »