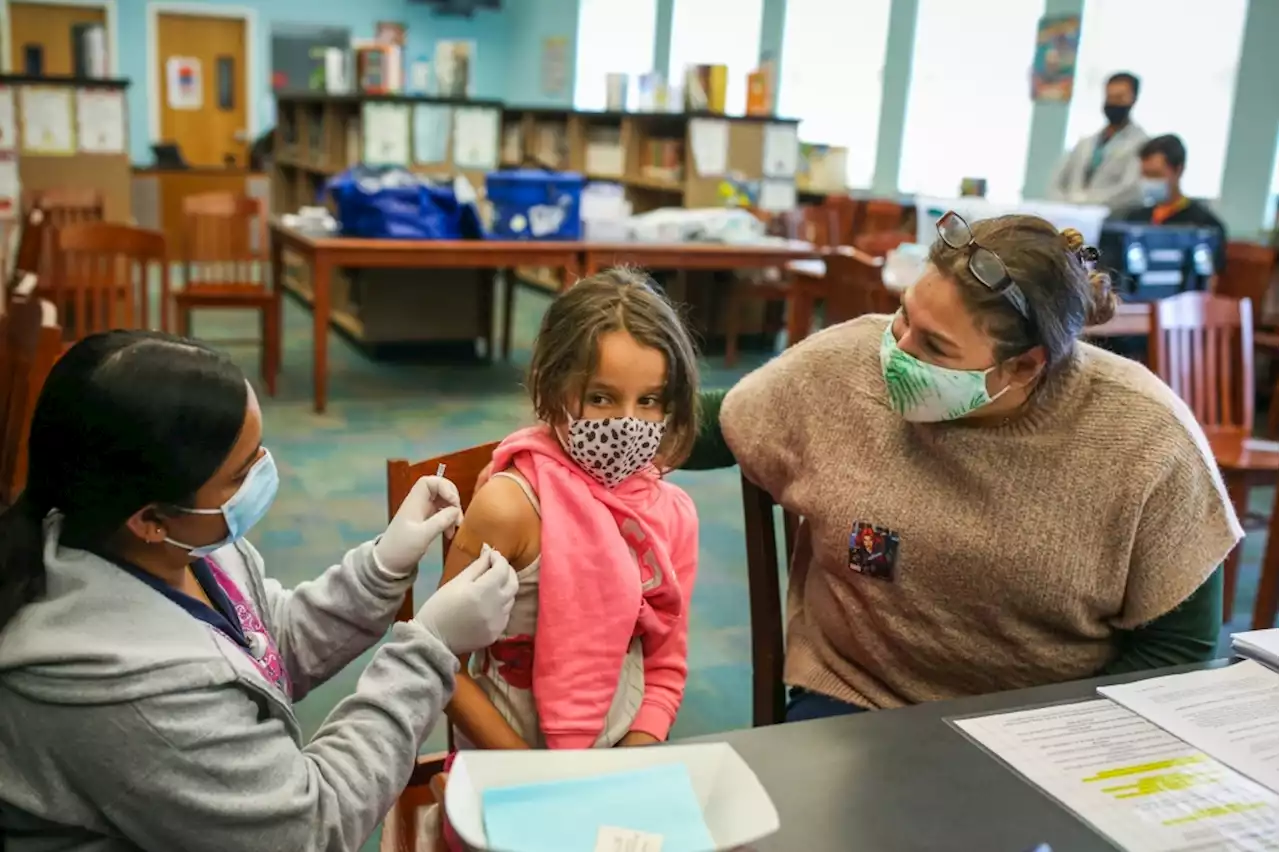
The CDC’s vaccine expert panel will consider whether to recommend the booster in young kids Thursday.
to recommend everyone wear masks indoors again. Since March the Bay Area has seen its case rate increase five-fold, while California’s has quadrupled.Yes, the booster is a third shot of the same Pfizer vaccine authorized for kids 5-11 last fall, which was given in two 10-microgram doses three weeks apart.Yes, the FDA authorized a single 30-microgram dose of Pfizer’s vaccine for those 12 and older on Jan. 3.That’s still unclear.
“With that third dose, you’re buying better protection against mild illness for the next 3 to 6 months,” Offit said. Dr. Monica Gandhi, an infectious disease specialist at UC-San Francisco, said antibodies are expected to increase after a booster dose but that does not necessarily translate to clinical benefit, and the Pfizer trial didn’t evaluate whether the booster shot prevented symptomatic COVID or severe disease.Offit said you could argue that it would make more sense to wait since the bulk of the protection against infection will be gone by then.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Lire la suite »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Lire la suite »
 FDA approves Pfizer COVID booster shots for kids 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11.
FDA approves Pfizer COVID booster shots for kids 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11.
Lire la suite »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
Lire la suite »
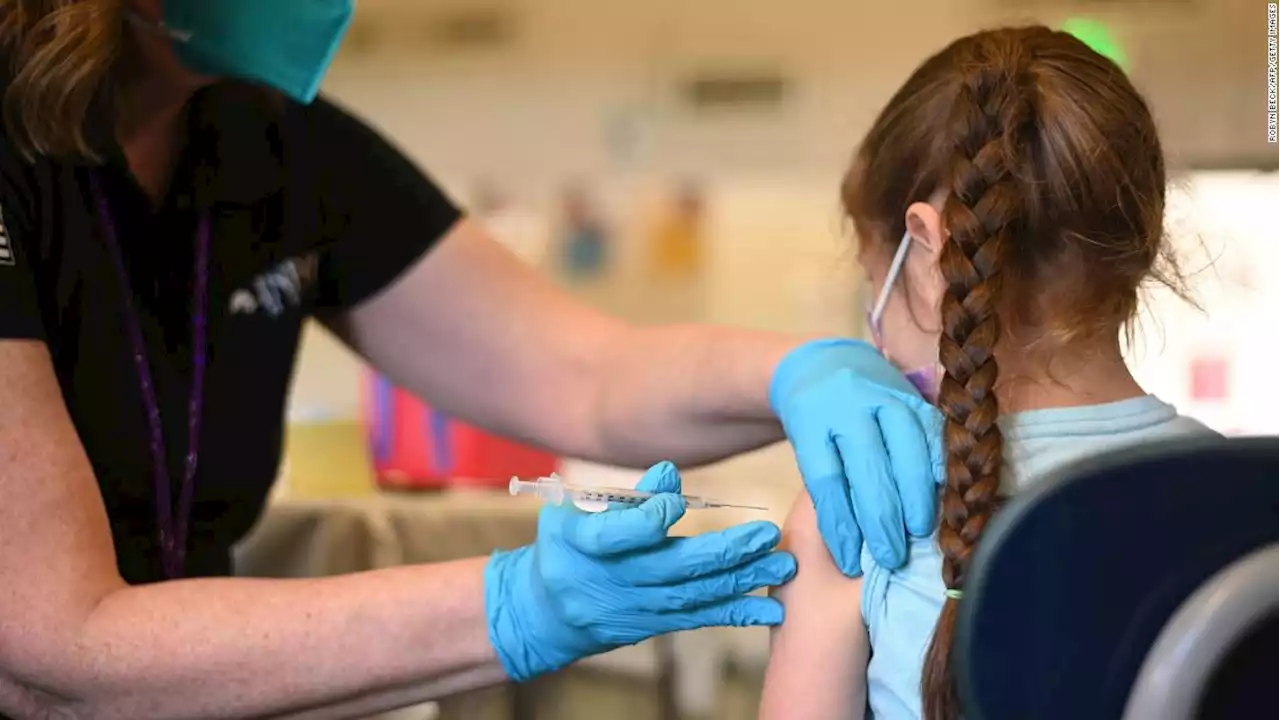 FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Lire la suite »
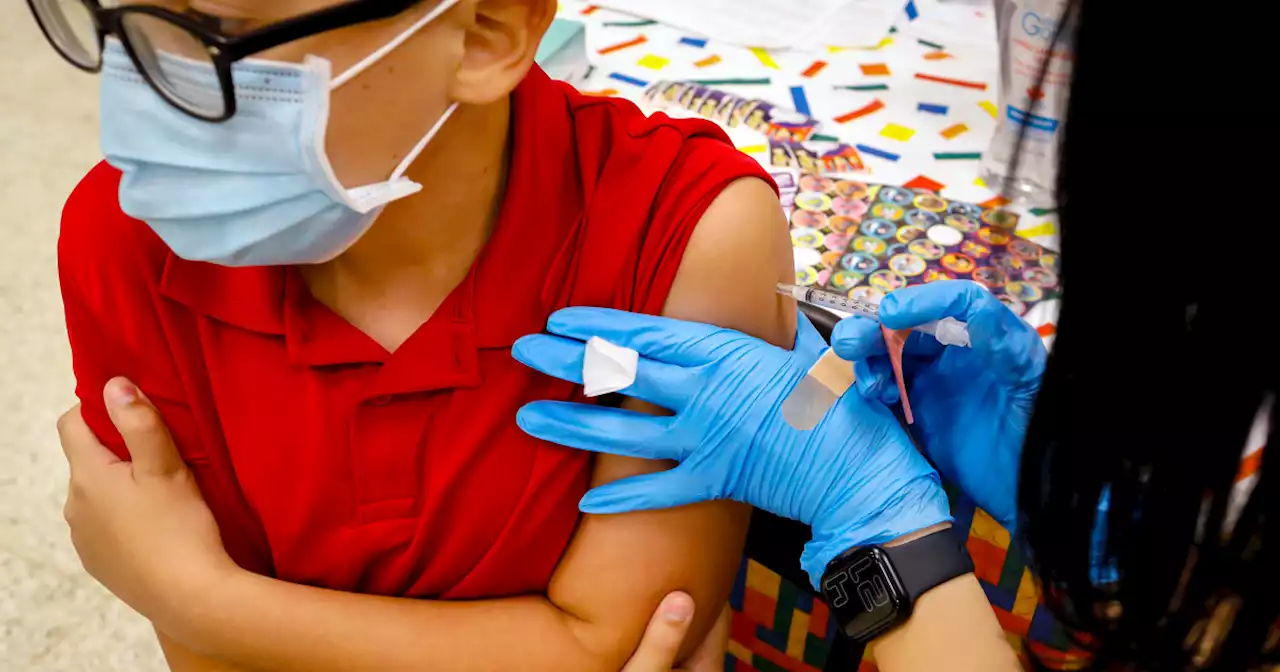 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Lire la suite »