Vaccine experts advising the CDC will discuss recommending Pfizer’s newly authorized Covid-19 booster for children ages 5 to 11
Review of Pfizer’s extra dose is one of the last steps before many pharmacies, doctors and other vaccination sites begin to offer the shots
Children under five in the U.S. aren’t yet eligible for a Covid-19 vaccine after efforts to get the shots to market have hit setbacks. WSJ’s Peter Loftus explains what we know about the vaccines and when they might become available. Photo composite: Todd JohnsonAn outside panel of experts for the Centers for Disease Control and Prevention on Thursday is scheduled to weigh whetherthe newly authorized Covid-19 booster shot from Pfizer Inc. and BioNTech SE.
The panel’s recommendations would guide the nation’s doctors, schools and local policy makers in what vaccine advice they give to parents of the 28 million U.S. children in the age group.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Lire la suite »
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Lire la suite »
 FDA authorizes COVID-19 booster for children ages 5 to 11The Food and Drug Administration (FDA) amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
FDA authorizes COVID-19 booster for children ages 5 to 11The Food and Drug Administration (FDA) amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
Lire la suite »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Lire la suite »
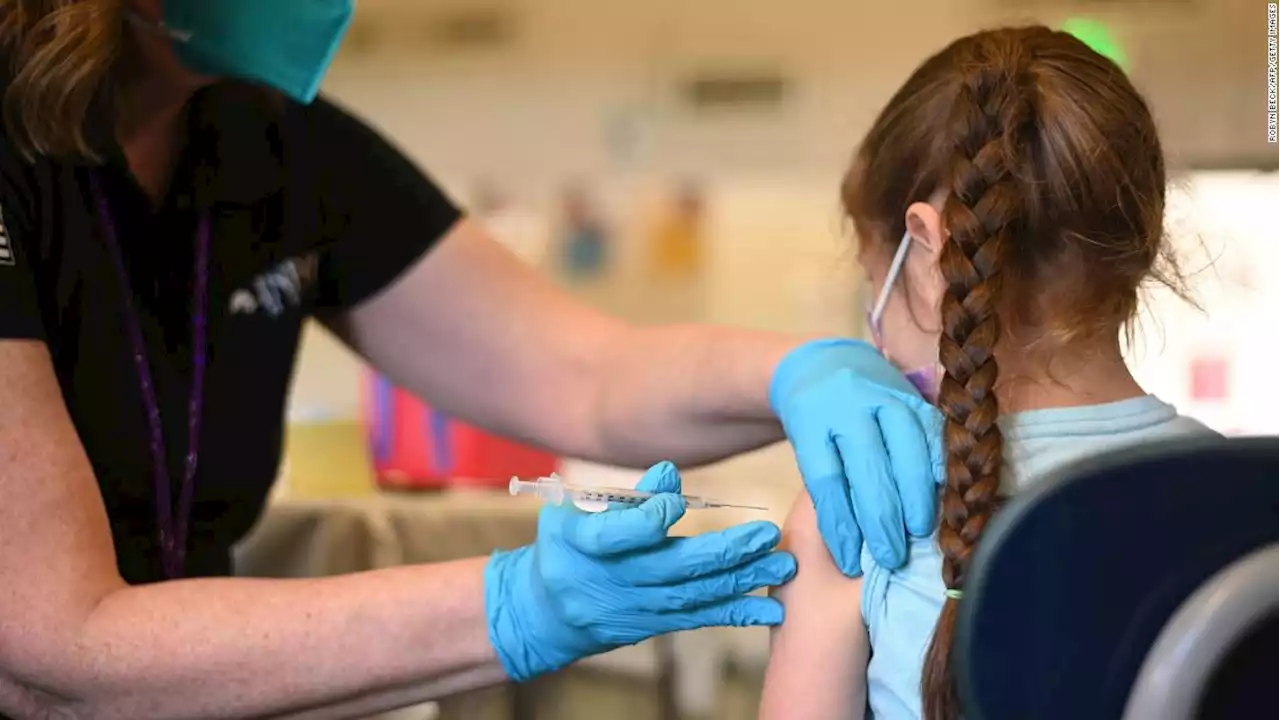 FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Lire la suite »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
Lire la suite »
