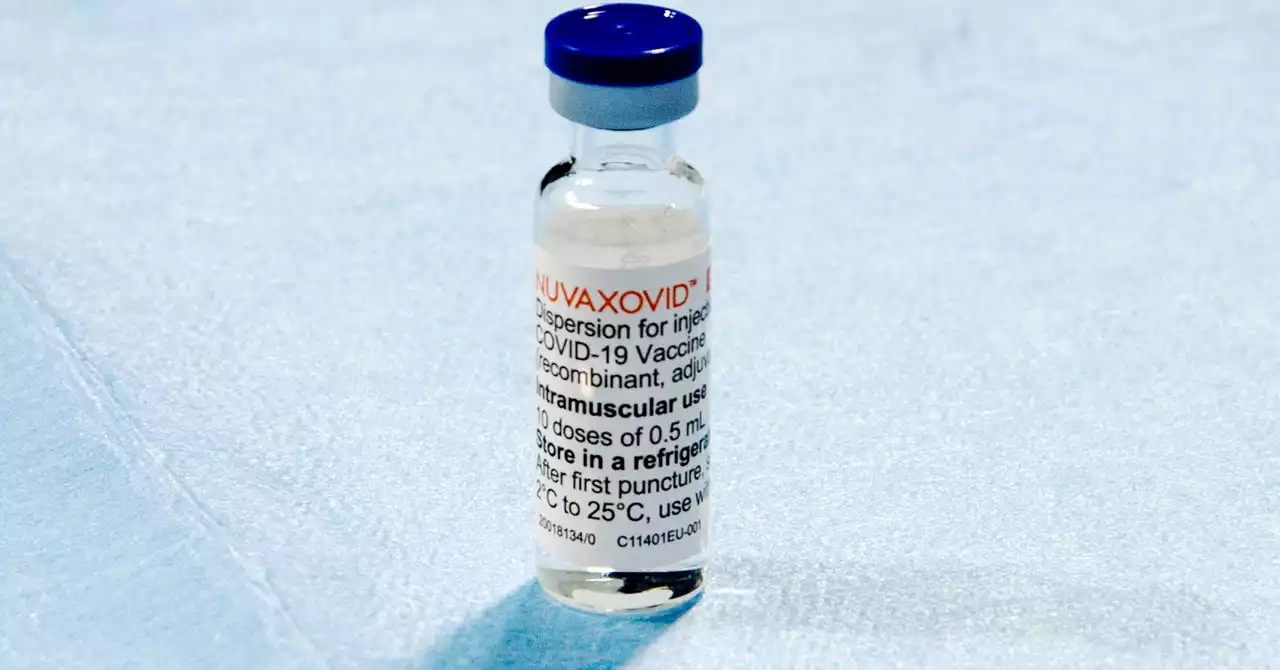
FDA advisers overwhelmingly endorsed the vaccine, which Novavax hopes will sway holdouts to finally get their shots.
will need to sign off on use before it becomes available.
Generally, the protein-subunit vaccine design is tried and trusted; it's already used in vaccines against flu, pertussis , and meningococcal infection, for example.Novavax leaned hard into the traditional design in its pitch to the FDA. Now that we're more than two years into the pandemic and mRNA vaccines are readily available in the US, most people who want to get vaccinated have already gotten their shots.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA panel recommends Novavax COVID-19 vaccine be the fourth allowed for use in USThe vaccine, which was supported by $1.8 billion in taxpayer funding, uses a more traditional approach than some other COVID-19 vaccines allowed in the USA.
FDA panel recommends Novavax COVID-19 vaccine be the fourth allowed for use in USThe vaccine, which was supported by $1.8 billion in taxpayer funding, uses a more traditional approach than some other COVID-19 vaccines allowed in the USA.
Lire la suite »
 FDA advisers back Novavax COVID shots as 4th US optionAmerican adults who haven’t yet gotten vaccinated against COVID-19 may soon get another choice, as advisers to the Food and Drug Administration on Tuesday backed a more traditional type of shot.
FDA advisers back Novavax COVID shots as 4th US optionAmerican adults who haven’t yet gotten vaccinated against COVID-19 may soon get another choice, as advisers to the Food and Drug Administration on Tuesday backed a more traditional type of shot.
Lire la suite »
FDA advisers greenlight Novavax COVID-19 vaccineA key U.S. FDA committee has recommended nearly unanimously that the agency grant an emergency authorization to a COVID19 vaccine from Novavax, opening the way for the first protein-based COVID-19 vaccine to become available to people in the U.S.
Lire la suite »
 FDA advisers back Novavax COVID-19 vaccine as 4th option for US adultsA more traditional kind of COVID-19 vaccine is a step closer to becoming the fourth option for U.S. adults.
FDA advisers back Novavax COVID-19 vaccine as 4th option for US adultsA more traditional kind of COVID-19 vaccine is a step closer to becoming the fourth option for U.S. adults.
Lire la suite »
 SoCal COVID cases rise as rest of nation plateaus, FDA authorizes Novavax vaccine for emergency useIn the Northeast, COVID-19 cases appear to be in a plateau, but what does that mean for Southern California?
SoCal COVID cases rise as rest of nation plateaus, FDA authorizes Novavax vaccine for emergency useIn the Northeast, COVID-19 cases appear to be in a plateau, but what does that mean for Southern California?
Lire la suite »
 Weight-loss drug called Wegovy receives FDA approvalWegovy — an under-the-skin injection that showed promise in weight-loss clinical trials — is the first drug approved for chronic weight management since 2014.
Weight-loss drug called Wegovy receives FDA approvalWegovy — an under-the-skin injection that showed promise in weight-loss clinical trials — is the first drug approved for chronic weight management since 2014.
Lire la suite »