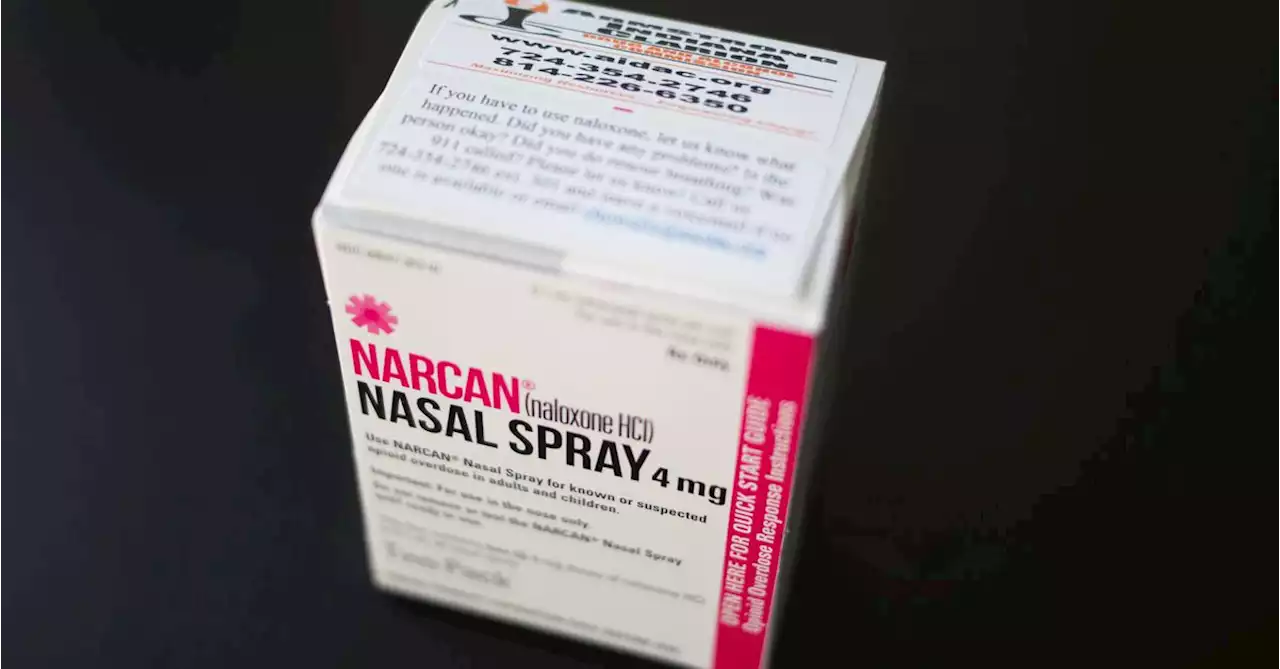
Contract drugmaker Emergent Biosolutions said on Tuesday its over-the-counter nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the U.S. health regulator.
said on Tuesday its over-the-counter nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the U.S. health regulator.
The agency will make its decision by March 29 and its priority review status puts Narcan on track to become the first naloxone-based drug to be sold over the counter, Benchmark analyst Robert Wasserman said. There are legal barriers limiting access to naloxone in some states, and even in others the drug is not always available to those most at risk of an overdose.
Government data estimates that there were more than 100,000 drug-related overdose deaths in the country during 2021, a near 15% increase from the year earlier.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Over-the-Counter Narcan to Reverse Overdoses May Be Available Early Next YearEmergent BioSolutions said Tuesday that the FDA will decide whether to approve its OTC nasal spray by late March 2023.
Over-the-Counter Narcan to Reverse Overdoses May Be Available Early Next YearEmergent BioSolutions said Tuesday that the FDA will decide whether to approve its OTC nasal spray by late March 2023.
Lire la suite »
 FDA change ushers in cheaper, easier-to-get hearing aidsThese over-the-counter hearing aids began hitting the market in October at prices that can be thousands of dollars lower than prescription hearing aids.
FDA change ushers in cheaper, easier-to-get hearing aidsThese over-the-counter hearing aids began hitting the market in October at prices that can be thousands of dollars lower than prescription hearing aids.
Lire la suite »
 FDA change ushers in cheaper, easier-to-get hearing aidsThese over-the-counter hearing aids began hitting the market in October at prices that can be thousands of dollars lower than prescription hearing aids.
FDA change ushers in cheaper, easier-to-get hearing aidsThese over-the-counter hearing aids began hitting the market in October at prices that can be thousands of dollars lower than prescription hearing aids.
Lire la suite »
 FDA change ushers in cheaper, easier-to-get hearing aidsThese over-the-counter hearing aids began hitting the market in October at prices that can be thousands of dollars lower than prescription hearing aids.
FDA change ushers in cheaper, easier-to-get hearing aidsThese over-the-counter hearing aids began hitting the market in October at prices that can be thousands of dollars lower than prescription hearing aids.
Lire la suite »
 FDA Takes Tougher Line on Fast-Tracked DrugsGSK and Roche have pulled drugs approved on an accelerated basis, while other drugmakers win speedy approvals only after starting confirmatory studies.
FDA Takes Tougher Line on Fast-Tracked DrugsGSK and Roche have pulled drugs approved on an accelerated basis, while other drugmakers win speedy approvals only after starting confirmatory studies.
Lire la suite »