Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Nurse practitioner Sarah Rauner fills a syringe with the Pfizer Covid-19 vaccine to be administered to children from 5-11 years old at the Beaumont Health offices in Southfield, Michigan on November 5, 2021.that the way for the shots – while awaiting data on a three-dose course, expected in March – could be cleared as soon as this month.
Early Pfizer data has shown the vaccine, administered to children at one-tenth the strength of the adult shot, is safe and produces an immune response. However, Pfizer announced last year that the two-dose shot proved to be less effective at preventing COVID-19 in kids ages 2-5, prompting regulators to encourage a third shot – administered at least two months after the second dose.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Lire la suite »
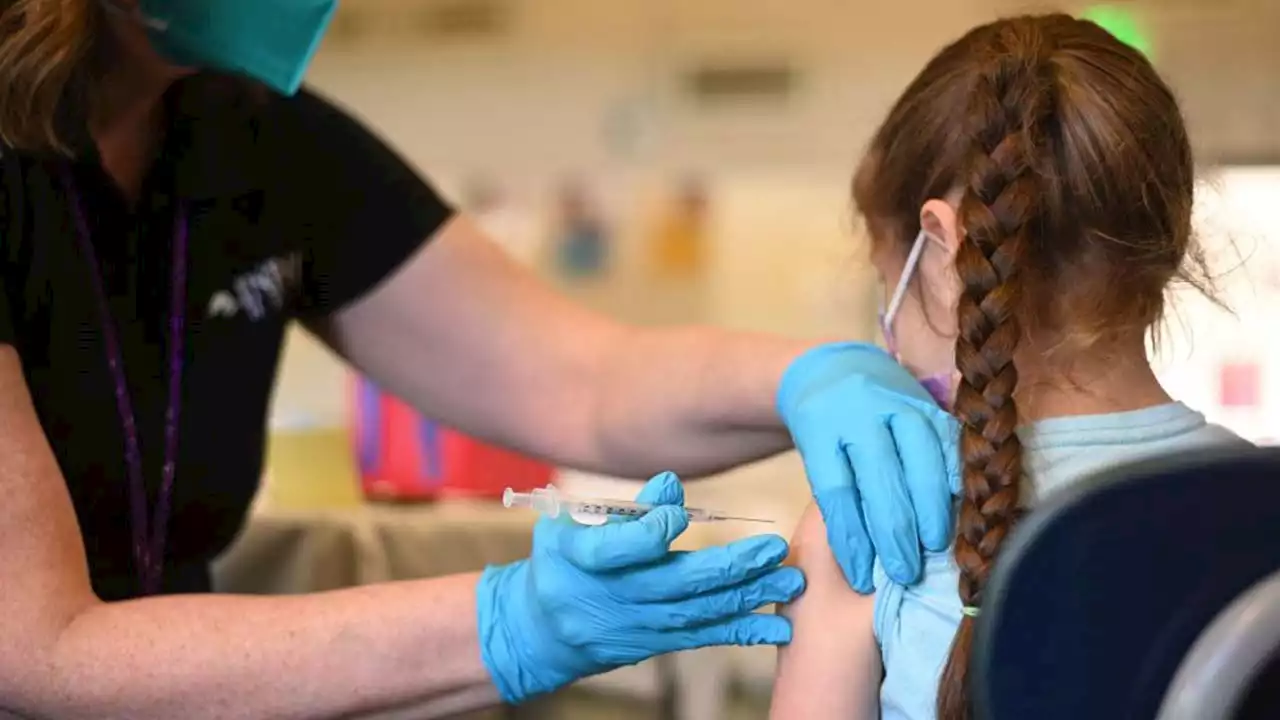 Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Lire la suite »
 Spotify to add advisories to podcasts discussing COVID-19Following protests of Spotify kicked off by Neil Young over the spread of COVID-19 vaccine misinformation, the music streaming service said that it will add content advisories before podcasts discussing the virus.
Spotify to add advisories to podcasts discussing COVID-19Following protests of Spotify kicked off by Neil Young over the spread of COVID-19 vaccine misinformation, the music streaming service said that it will add content advisories before podcasts discussing the virus.
Lire la suite »
COVID-19: endemic doesn’t mean harmlessRosy assumptions endanger public health — policymakers must act now to shape the years to come.
Lire la suite »
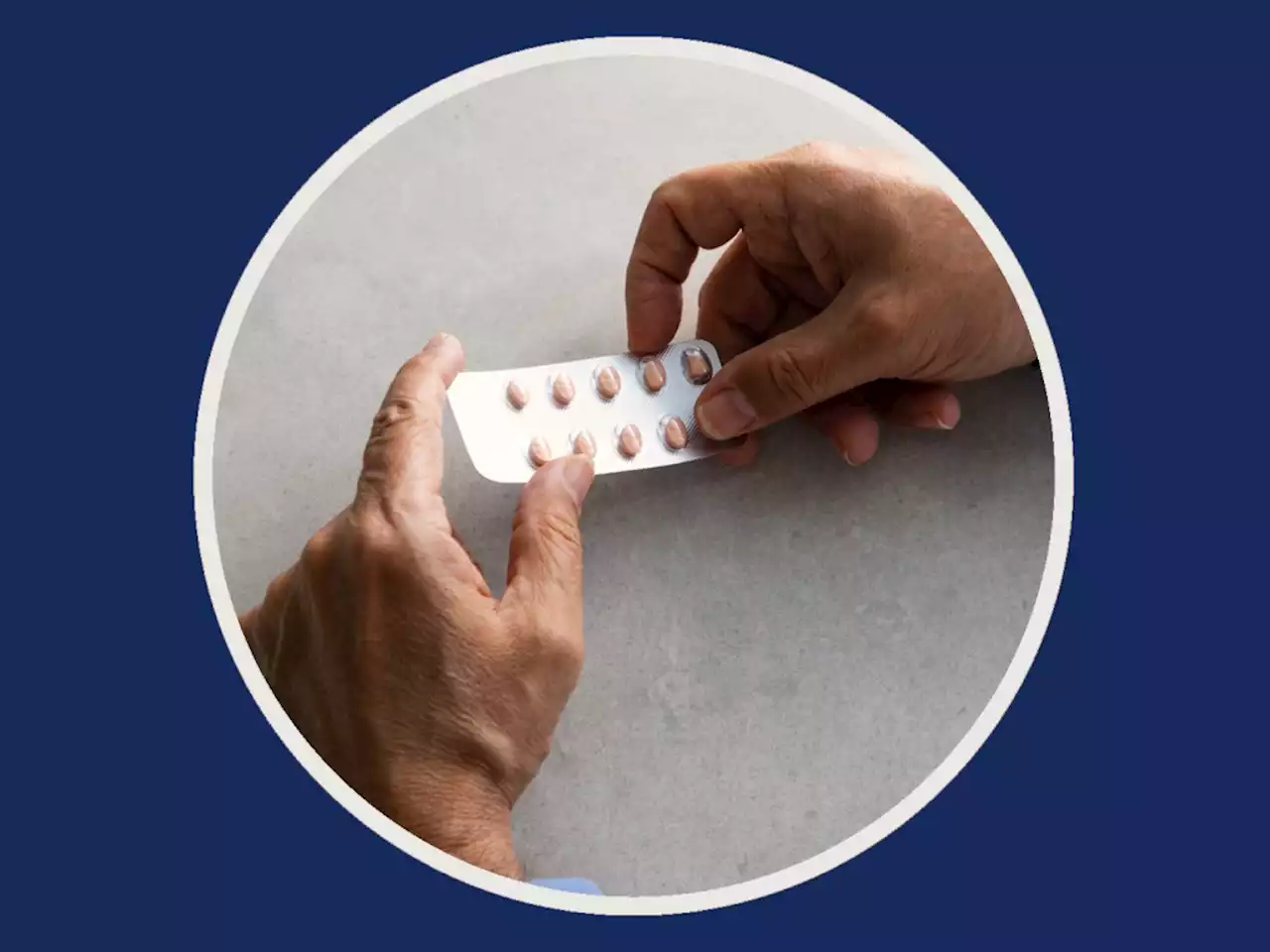 What You Need to Know About the COVID-19 Pill Available Right NowThis is everything you need to know about the COVID-19 pill and who is eligible to take it.
What You Need to Know About the COVID-19 Pill Available Right NowThis is everything you need to know about the COVID-19 pill and who is eligible to take it.
Lire la suite »
