Pfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
Charlotte Allerton, Pfizer's chief scientific officer, said COVID-19 has "the potential to remain a global health concern for years to come".to generate about $22 billion in revenue this year. Pfizer has been making various deals to boost its portfolio. This year, it has announced acquisitions of Biohaven Pharmaceutical Holding Co
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Lire la suite »
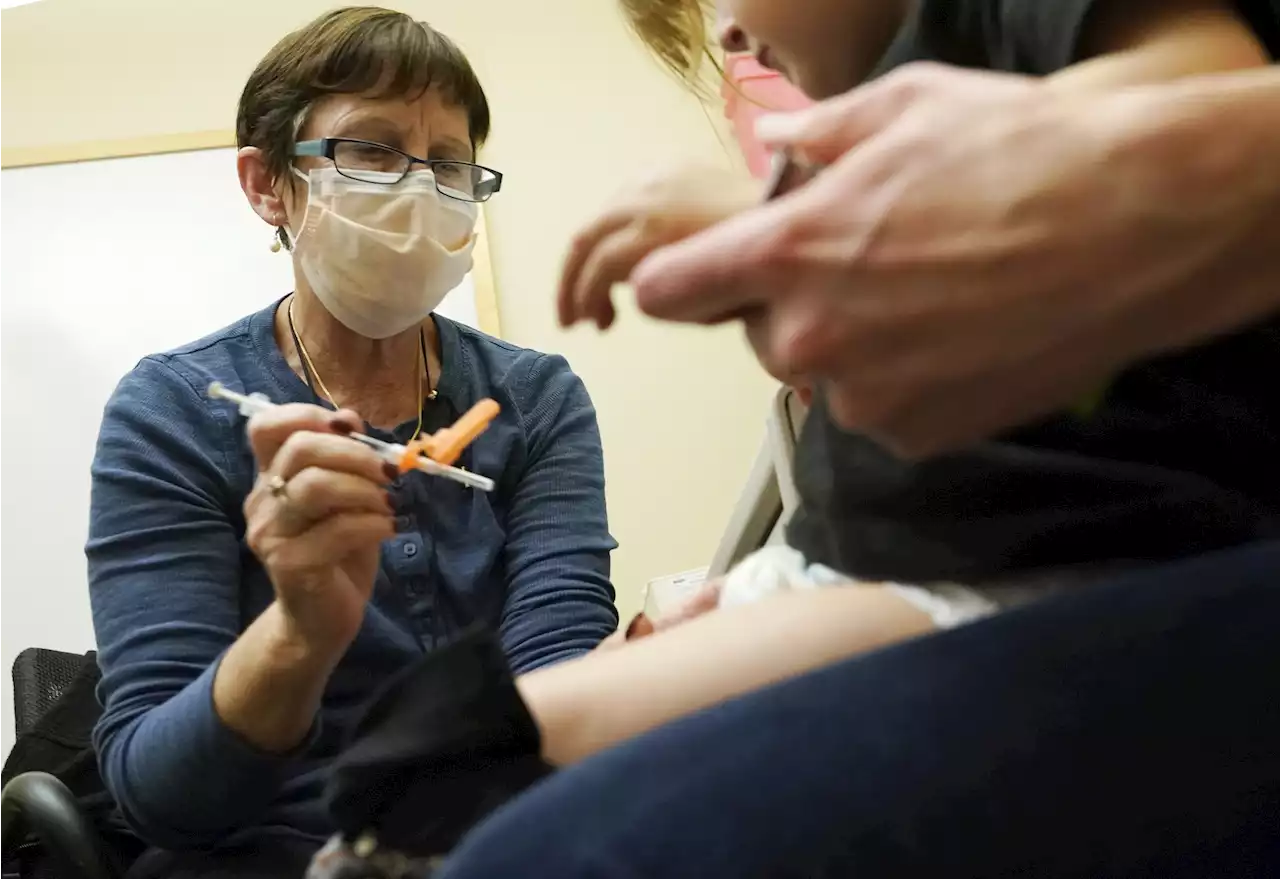 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Lire la suite »
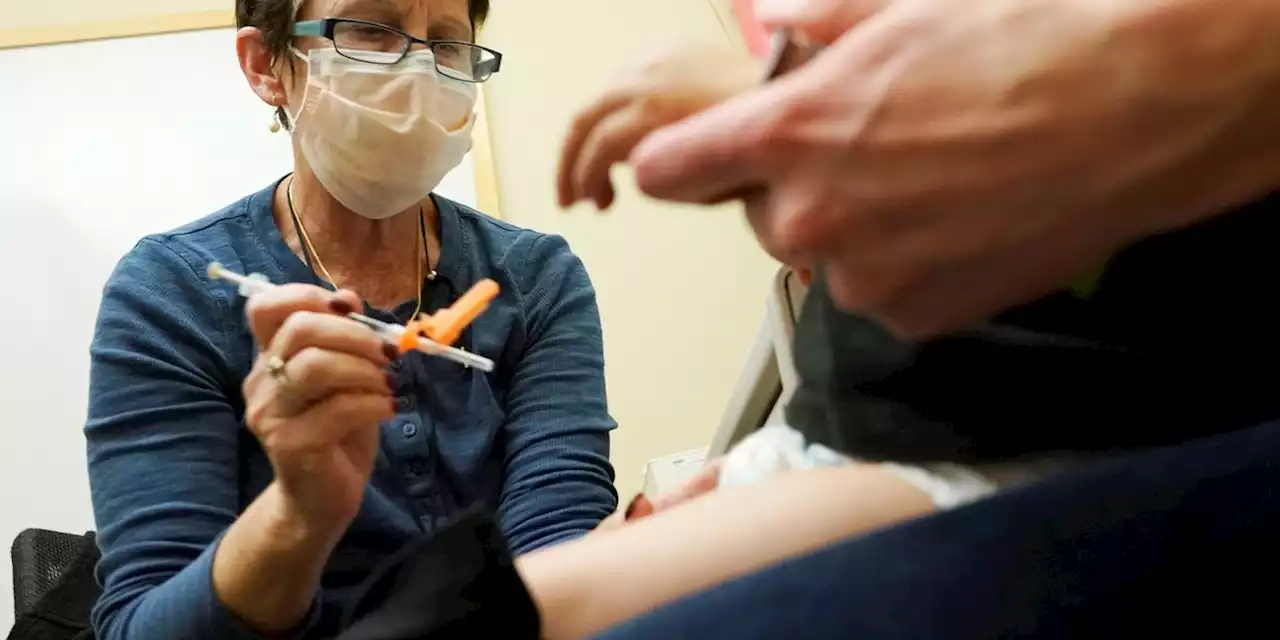 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Lire la suite »
 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Lire la suite »
 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots.
Lire la suite »
