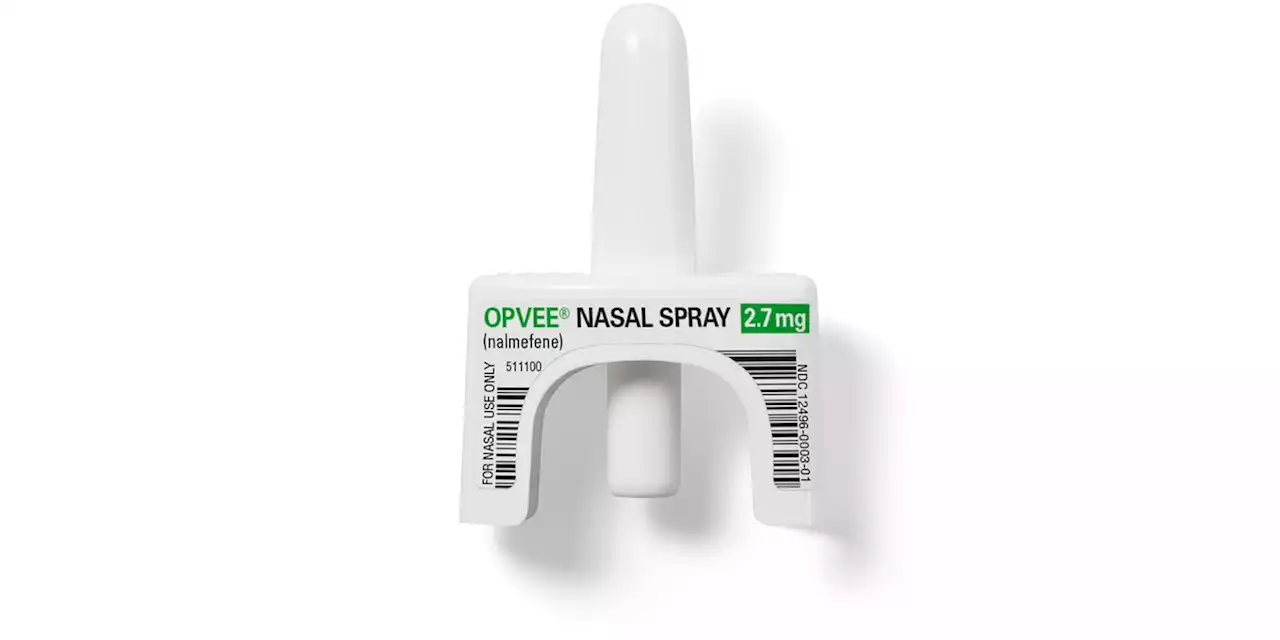
In studies funded by the federal government, Opvee achieved similar recovery results to Narcan, the leading brand of naloxone nasal spray.
Both work by blocking the effects of opioids in the brain, which can restore normal breathing and blood pressure in people who have recently overdosed.
Opvee was developed by Opiant Pharmaceuticals, which was recently acquired by rival Indivior, maker of several medications for opioid addiction. Indivior expects to launch Opvee in October at the earliest. Scientists at the National Institutes of Health worked with pharmaceutical researchers on a nasal spray version of nalmefene that would quickly resuscitate users, while also protecting them from relapse. Testing and development was funded by more than $18 million in grants from the U.S. government’s Biomedical Advanced Research and Development Authority and the NIH, which also helped design the studies.
“The risk of long-lasting withdrawal is very real and we try to avoid it,” said Nelson, an emergency medicine physician and former adviser to the FDA on opioids.“We’re not suffering from a naloxone shortage where we need to use an alternative,” he said. “We have plenty of it and it works perfectly well.”
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalOpvee is similar to naloxone, the life-saving drug that has been used for decades to quickly counter overdoses of heroin, fentanyl and prescription painkillers.
New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalOpvee is similar to naloxone, the life-saving drug that has been used for decades to quickly counter overdoses of heroin, fentanyl and prescription painkillers.
Lire la suite »
 New Nasal Spray to Reverse Fentanyl and Other Opioid Overdoses Gets FDA ApprovalThe drug will be available via prescription and is approved for patients 12 and older.
New Nasal Spray to Reverse Fentanyl and Other Opioid Overdoses Gets FDA ApprovalThe drug will be available via prescription and is approved for patients 12 and older.
Lire la suite »
 New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalOpvee is similar to naloxone, the life-saving drug that has been used for decades to quickly counter overdoses of heroin, fentanyl and prescription painkillers.
New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalOpvee is similar to naloxone, the life-saving drug that has been used for decades to quickly counter overdoses of heroin, fentanyl and prescription painkillers.
Lire la suite »
 New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalU.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation’s drug crisis.
New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalU.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation’s drug crisis.
Lire la suite »
 Veozah, new menopause drug for hot flashes, wins FDA approval“It’s going to be completely game changing for a lot of women,” one endocrinologist said.
Veozah, new menopause drug for hot flashes, wins FDA approval“It’s going to be completely game changing for a lot of women,” one endocrinologist said.
Lire la suite »
 California plans to spend more on Narcan, but it could lose workers who hand it outCommunity groups that routinely hand out the lifesaving medication fear their workers could soon be facing layoffs with the disappearance of a key grant.
California plans to spend more on Narcan, but it could lose workers who hand it outCommunity groups that routinely hand out the lifesaving medication fear their workers could soon be facing layoffs with the disappearance of a key grant.
Lire la suite »