The FDA is warning consumers that three lots of milk of magnesia are being recalled due to possible bacterial contamination.
Individuals with a compromised immune system have a higher probability of developing a widespread, potentially life-threatening infection when ingesting or otherwise orally exposed to products contaminated by micro-organisms, the FDA says.
Plastikon Healthcare, which makes milk of magnesia, says some lots of its oral suspension product, as well as acetaminophen and magnesium, may be affected.The product is packaged for institutional use and is sold to clinics and hospitals nationwide in single-use cups with a foil lid. The affected lots were distributed to Major Pharmaceuticals Distribution Center between 5/1/2020 and 6/28/2021, who shipped to hospitals, nursing homes, and clinics nationwide.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA: Nationwide recall of Milk of Magnesia, other medicines due to possible contaminationPlastikon Healthcare, based in Kansas, is voluntarily recalling a select number of products sold nationally due to possible bacteria contamination.
FDA: Nationwide recall of Milk of Magnesia, other medicines due to possible contaminationPlastikon Healthcare, based in Kansas, is voluntarily recalling a select number of products sold nationally due to possible bacteria contamination.
Lire la suite »
 FDA: Nationwide recall of Milk of Magnesia, other medicines due to possible contaminationPlastikon Healthcare, based in Kansas, is voluntarily recalling a select number of products sold nationally due to possible bacteria contamination.
FDA: Nationwide recall of Milk of Magnesia, other medicines due to possible contaminationPlastikon Healthcare, based in Kansas, is voluntarily recalling a select number of products sold nationally due to possible bacteria contamination.
Lire la suite »
 FDA details problems at Abbott plant behind recalled baby formulaThe Food and Drug Administration posted its initial inspection findings from the Abbott plant that’s been tied to several infant hospitalizations, including two deaths, due to a rare bacterial infection.
FDA details problems at Abbott plant behind recalled baby formulaThe Food and Drug Administration posted its initial inspection findings from the Abbott plant that’s been tied to several infant hospitalizations, including two deaths, due to a rare bacterial infection.
Lire la suite »
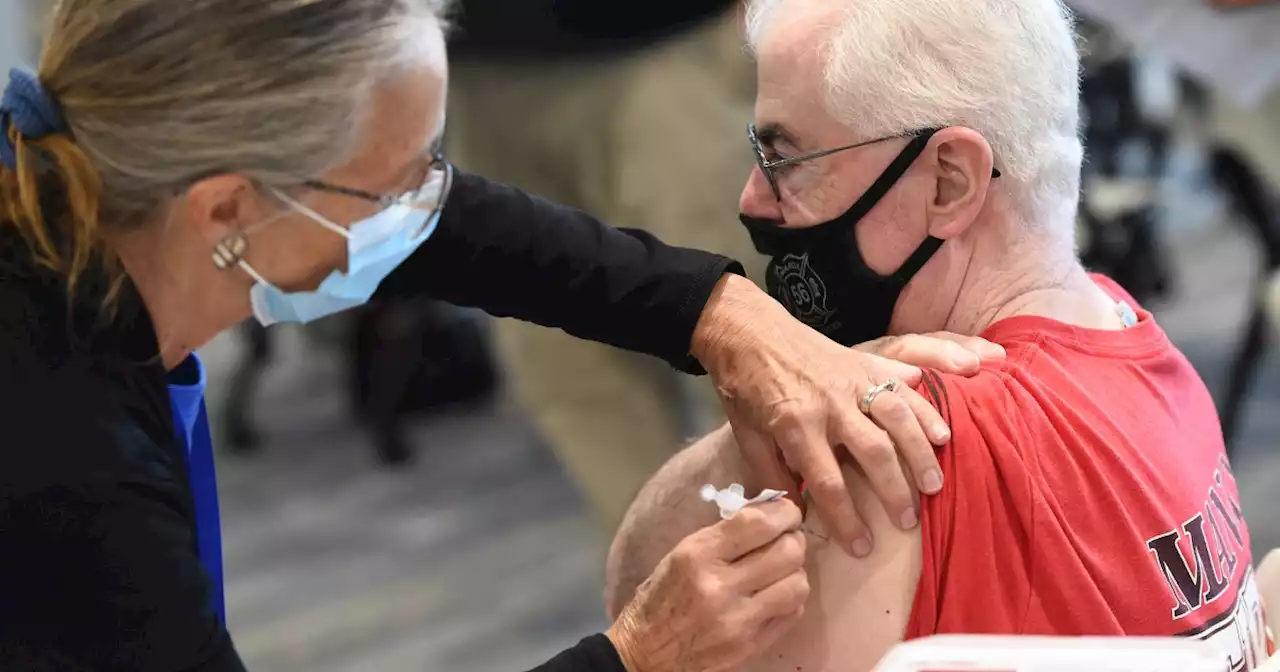 FDA expected to authorize a second Covid booster shot next weekFDA expected to authorize a second Covid booster shot next week. The New York Times, which first reported the news, said late Friday that the Biden administration planned to offer second boosters to Americans age 50 and older.
FDA expected to authorize a second Covid booster shot next weekFDA expected to authorize a second Covid booster shot next week. The New York Times, which first reported the news, said late Friday that the Biden administration planned to offer second boosters to Americans age 50 and older.
Lire la suite »
 FDA expected to authorize second coronavirus booster for 50 and olderThe FDA is poised to authorize a second coronavirus vaccine booster for anyone 50 and older. It would provide an extra layer of protection amid concerns Europe’s rise in infections from an omicron subvariant could hit the United States.
FDA expected to authorize second coronavirus booster for 50 and olderThe FDA is poised to authorize a second coronavirus vaccine booster for anyone 50 and older. It would provide an extra layer of protection amid concerns Europe’s rise in infections from an omicron subvariant could hit the United States.
Lire la suite »
 FDA Reviews ALS Drug with Modest DataThe FDA meets next week to publicly review evidence from a small, mid-stage study of AMX0035, a drug made by Amylyx Pharmaceuticals, which shows modest effects on patients’ decline.
FDA Reviews ALS Drug with Modest DataThe FDA meets next week to publicly review evidence from a small, mid-stage study of AMX0035, a drug made by Amylyx Pharmaceuticals, which shows modest effects on patients’ decline.
Lire la suite »
