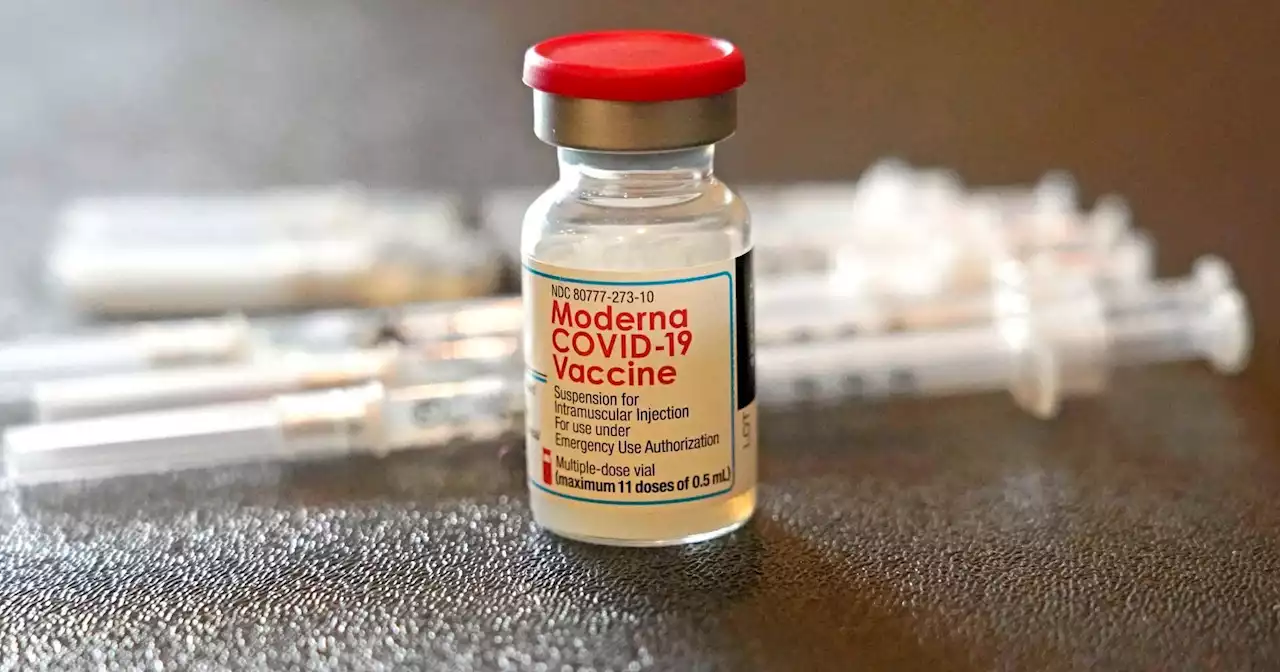
Moderna asks U.S. regulators to authorize its COVID-19 vaccine for children younger than 6, which may make shots available by summer.
Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna submitted data to the Food and Drug Administration that it hopes will prove two low-dose shots can protect babies, toddlers and preschoolers -- albeit not as effectively during the omicron surge as earlier in the pandemic. Now, only children ages 5 or older can be vaccinated in the U.S., using rival Pfizer's vaccine, leaving 18 million younger tots unprotected.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Lire la suite »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Lire la suite »
 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Lire la suite »
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Lire la suite »
 Moderna seeks to be 1st with COVID shots for littlest kidsModerna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna seeks to be 1st with COVID shots for littlest kidsModerna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Lire la suite »
 Administration expands availability of COVID-19 antiviral pillPresident Joe Biden’s administration is taking steps to expand availability of the life-saving COVID-19 antiviral treatment Paxlovid.
Administration expands availability of COVID-19 antiviral pillPresident Joe Biden’s administration is taking steps to expand availability of the life-saving COVID-19 antiviral treatment Paxlovid.
Lire la suite »