U.S. health officials currently recommend a primary series of two doses of the Moderna vaccine and a booster dose months later.
Moderna said its request for an additional dose was based on “recently published data generated in the United States and Israel following the emergence of
Omicron.”asked U.S. regulators to authorize an additional booster dose of their COVID-19 vaccine for seniors, saying data from Israel suggests older adults would benefit.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Moderna seeks FDA authorization for 4th dose of COVID shotJUST IN: Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna seeks FDA authorization for 4th dose of COVID shotJUST IN: Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Lire la suite »
 Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Lire la suite »
 Moderna seeks FDA authorization for 4th dose of COVID shotWASHINGTON (AP) — Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer's request earlier this week for the regulator to approve a booster shot for all seniors.
Moderna seeks FDA authorization for 4th dose of COVID shotWASHINGTON (AP) — Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer's request earlier this week for the regulator to approve a booster shot for all seniors.
Lire la suite »
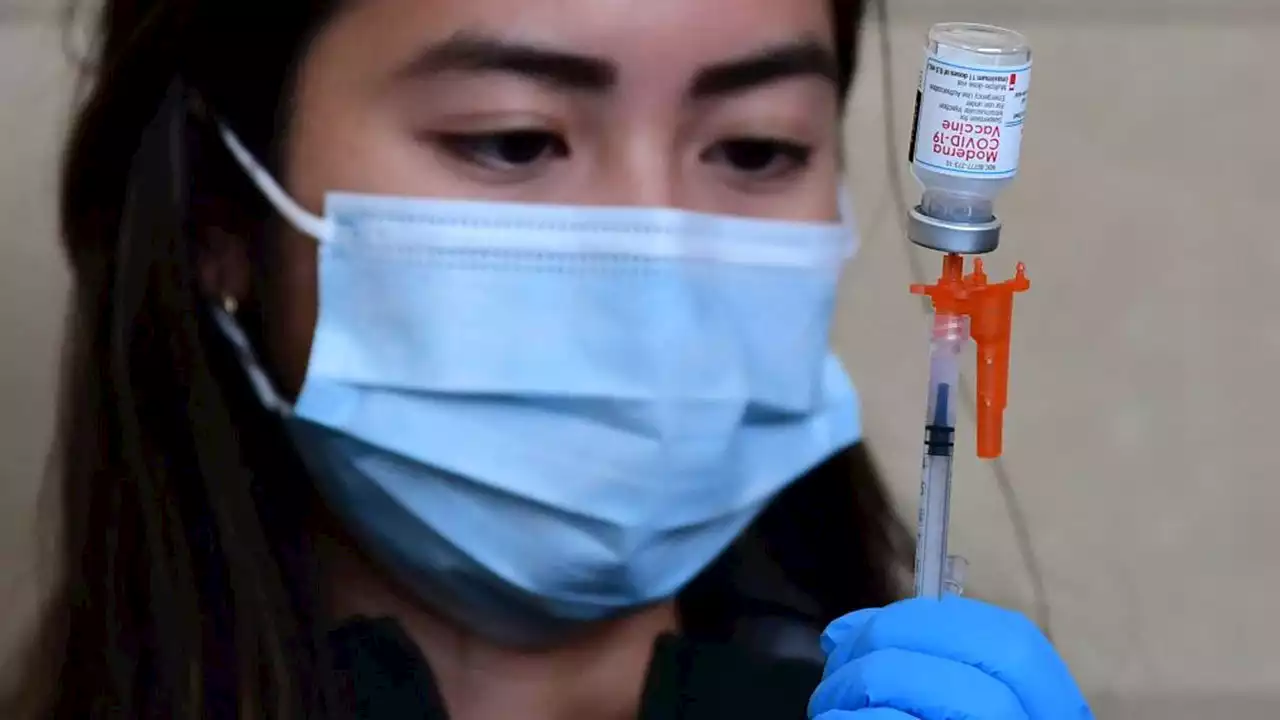 Moderna asks FDA to add 4th COVID-19 vaccine booster dose for all adults to EUADrugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna asks FDA to add 4th COVID-19 vaccine booster dose for all adults to EUADrugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Lire la suite »
 Moderna Seeks FDA Authorization for 4th Dose of COVID ShotDrugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna Seeks FDA Authorization for 4th Dose of COVID ShotDrugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Lire la suite »