Moderna released study results today showing its new Omicron-specific booster increased antibodies against the coronavirus by a factor of 5, even against some of the newer and more worrisome variants.
The booster in development contains both Moderna’s original COVID-19 vaccine and one specifically designed to target the more recent BA.4 and BA.5 Omicron subvariants.
The announcement comes at a time when the proportion of BA.4 and BA.5 subvariants in the United States is growing. These two strains now account for about 35% of those circulating in the U.S., according toBA.4 and BA.5"represent an emergent threat to global public health," Stéphane Bancel, chief executive officer of Moderna, stated in a.
The combination vaccine was not as strong against all Omicron variants though, showing that antibodies against BA.4 and BA.5 were approximately three times lower than against the older BA.1 variant. The late-stage study shows that 1 month after giving the shot to people already vaccinated and boosted, this new booster created"potent neutralizing antibody responses" against BA.4 and BA.5 in all who got the shot, regardless of prior infection, the company
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
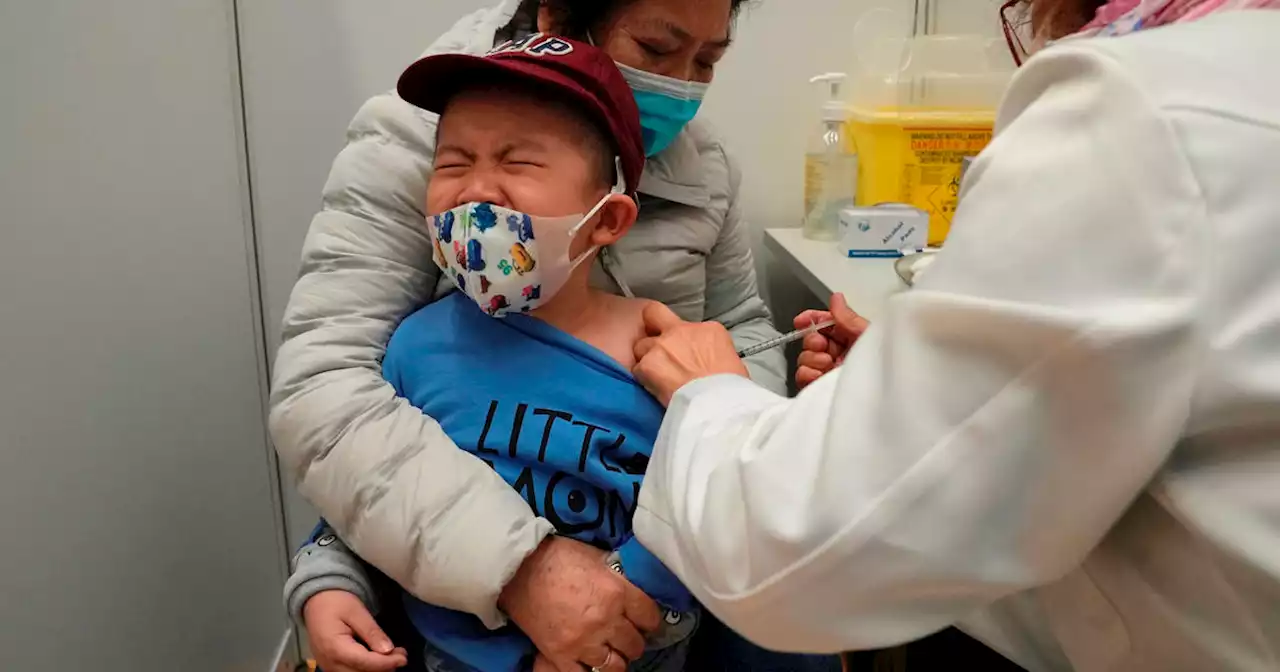 Bay Area kids under 5 to begin getting COVID vaccine following CDC, FDA approvalBay Area children under age 5 will have access to the COVID-19 vaccine for the first time this week following federal regulators' approval of the Pfizer-BioNTech and Moderna vaccines.
Bay Area kids under 5 to begin getting COVID vaccine following CDC, FDA approvalBay Area children under age 5 will have access to the COVID-19 vaccine for the first time this week following federal regulators' approval of the Pfizer-BioNTech and Moderna vaccines.
Lire la suite »
 FDA authorizes COVID-19 vaccines for children as young as 6 months - New York Amsterdam NewsThe U.S. Food and Drug Administration (FDA) authorized emergency use of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include use n children down to 6 months of age. [...]
FDA authorizes COVID-19 vaccines for children as young as 6 months - New York Amsterdam NewsThe U.S. Food and Drug Administration (FDA) authorized emergency use of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include use n children down to 6 months of age. [...]
Lire la suite »
 COVID vaccinations for the youngest San Diegans are underwayCOVID-19 vaccines are finally available for kids ages 6 months to 5 years old following CDC and FDA approvals.
COVID vaccinations for the youngest San Diegans are underwayCOVID-19 vaccines are finally available for kids ages 6 months to 5 years old following CDC and FDA approvals.
Lire la suite »
 Shots for tots: COVID vaccinations start for little US kids“With this vaccine, that’ll be his best shot at going back to normal and having a normal childhood.”
Shots for tots: COVID vaccinations start for little US kids“With this vaccine, that’ll be his best shot at going back to normal and having a normal childhood.”
Lire la suite »
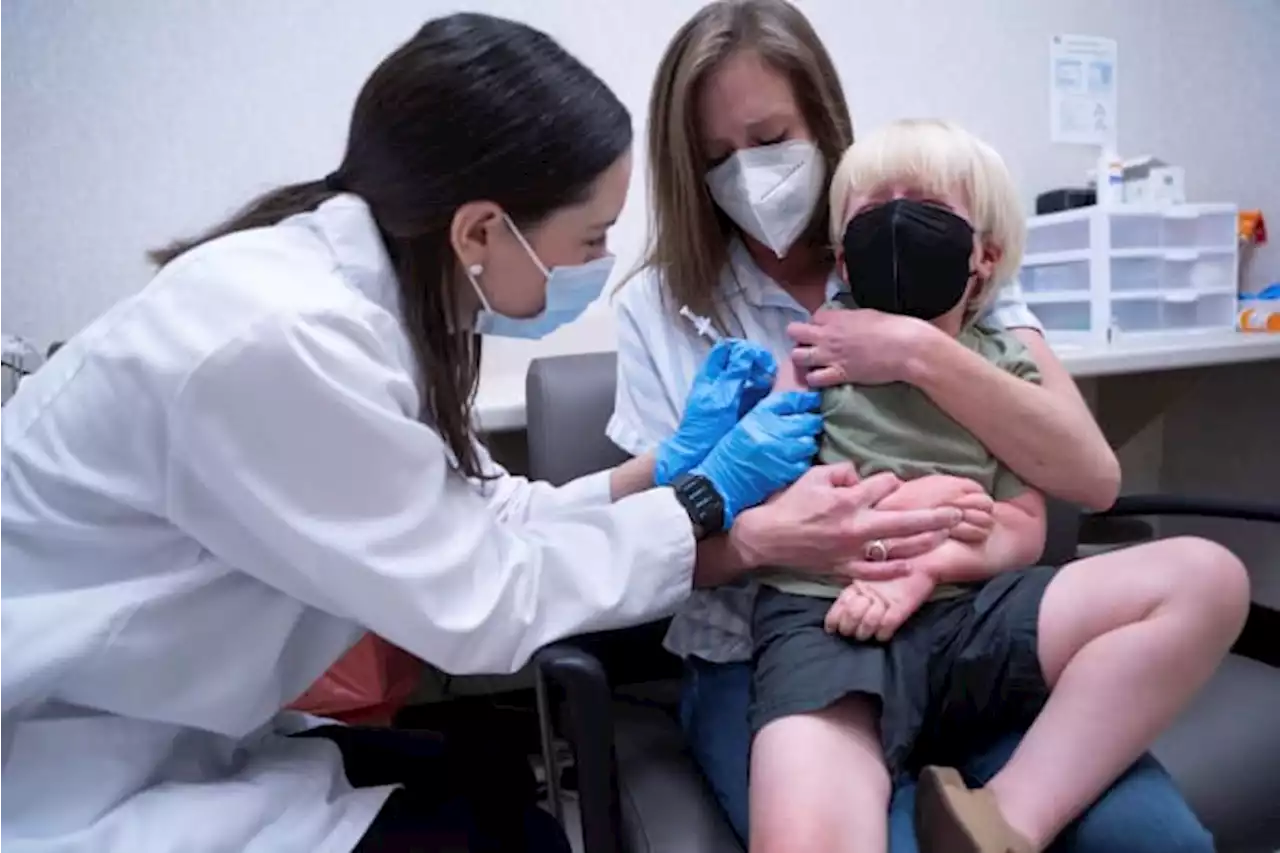 Shots for tots: COVID vaccinations start for little US kidsThe nation’s infants, toddlers and preschoolers are finally getting their chance at COVID-19 vaccination as the U.S. rolls out shots for tots this week.
Shots for tots: COVID vaccinations start for little US kidsThe nation’s infants, toddlers and preschoolers are finally getting their chance at COVID-19 vaccination as the U.S. rolls out shots for tots this week.
Lire la suite »
