Biogen drug, the first in decades approved to treat the disease, has sparked concerns about its high cost and efficacy.
Medicare officials are proposing to restrict coverage of a controversial Alzheimer's drug, Aduhelm, to participants in the federal health insurance program who are enrolled in qualifying clinical trials., the Centers for Medicare & Medicaid Services said Tuesday that it based its proposal on an analysis of the benefits and risks of the drug, which has raised concerns about Aduhelm's high cost and efficacy.
"While there may be the potential for promise with this treatment, there is also the potential for harm to patients," said Dr. Lee Fleisher, chief medical officer at CMS and director of the Center for Clinical Standards and Quality, in the statement."This harm may range from headaches, dizziness and falls, to other potentially serious complications such as brain bleeds."
The agency said will make a final decision on covering the drug by April 11. Medicare's decision on whether to pay for Aduhelm is important because it affects whether health insurers will cover the cost. The Food and Drug Administration approved Aduhelm last June, the first drug to cleared by government health officials for treating Alzheimer's in nearly two decades. But the treatment has also sparked criticism, with the FDA's outside panel of neurological experts
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 U.S. Medicare plans to cover Biogen Alzheimer's drug only for trial patientsThe U.S. government Medicare program on Tuesday said it plans to cover Alzheimer's treatments including Biogen Inc's Aduhelm but will require patients to be enrolled in a clinical trial, potentially limiting access.
U.S. Medicare plans to cover Biogen Alzheimer's drug only for trial patientsThe U.S. government Medicare program on Tuesday said it plans to cover Alzheimer's treatments including Biogen Inc's Aduhelm but will require patients to be enrolled in a clinical trial, potentially limiting access.
Lire la suite »
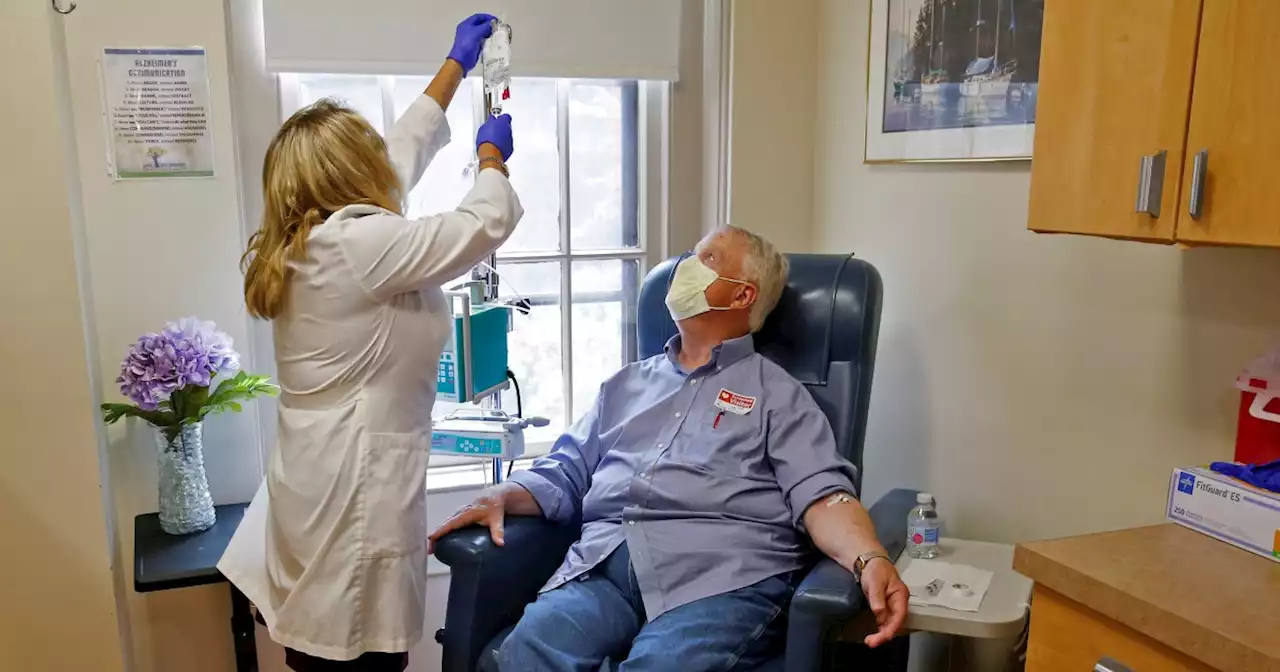 Medicare plans to pay for controversial Alzheimer’s drug, if patients enroll in trialsMedicare plans to provide insurance coverage for Aduhelm, a controversial Alzheimer’s treatment from drugmaker Biogen.
Medicare plans to pay for controversial Alzheimer’s drug, if patients enroll in trialsMedicare plans to provide insurance coverage for Aduhelm, a controversial Alzheimer’s treatment from drugmaker Biogen.
Lire la suite »
 Medicare proposes coverage limits for Alzheimer’s drug AduhelmMounting premium pressures contributed to the determination.
Medicare proposes coverage limits for Alzheimer’s drug AduhelmMounting premium pressures contributed to the determination.
Lire la suite »
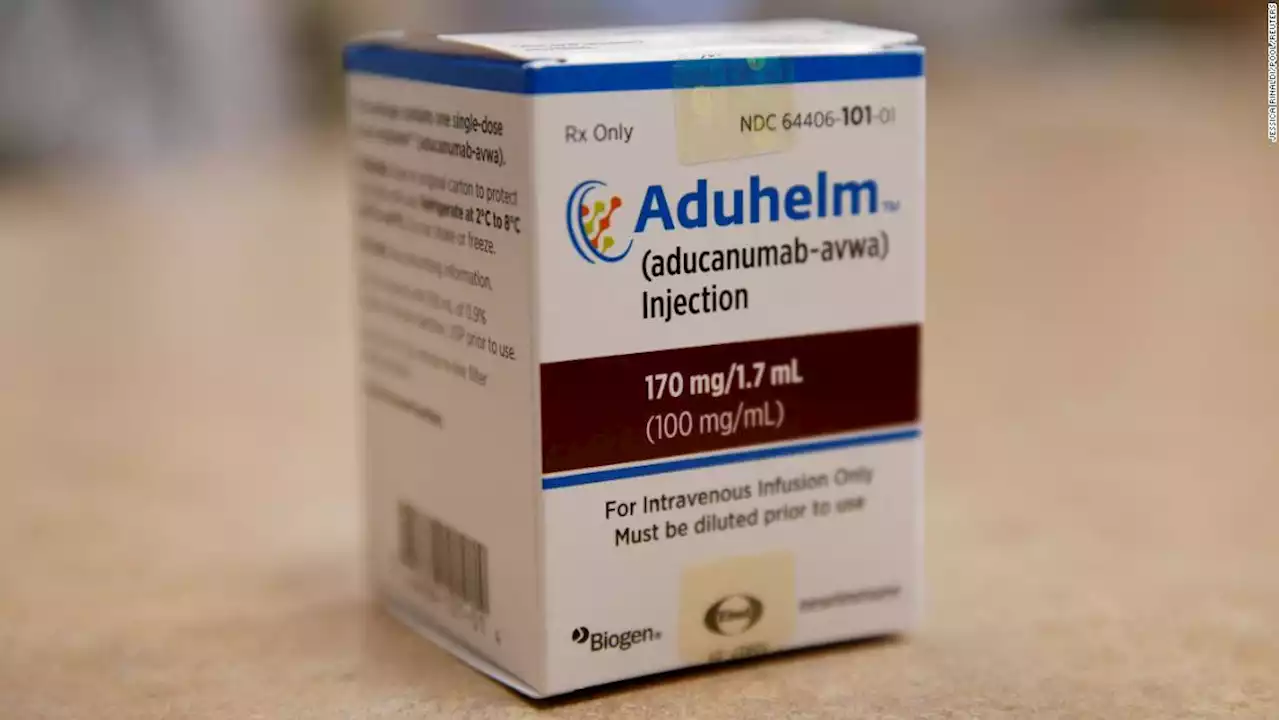 Medicare proposes to cover controversial Alzheimer's drug with restrictionsMedicare proposes covering a costly and controversial Alzheimer's drug, with some restrictions. It could have vast consequences for patients and enrollees.
Medicare proposes to cover controversial Alzheimer's drug with restrictionsMedicare proposes covering a costly and controversial Alzheimer's drug, with some restrictions. It could have vast consequences for patients and enrollees.
Lire la suite »
 Medicare limits coverage of Alzheimer’s drug with $28,000-a-year price tagBiogen’s initial launch price of $56,000 a year for Aduhelm led to an increase of nearly $22 in Medicare’s monthly “Part B” premium for outpatient care, the largest ever in dollar terms but not percentage-wise.
Medicare limits coverage of Alzheimer’s drug with $28,000-a-year price tagBiogen’s initial launch price of $56,000 a year for Aduhelm led to an increase of nearly $22 in Medicare’s monthly “Part B” premium for outpatient care, the largest ever in dollar terms but not percentage-wise.
Lire la suite »
 Medicare Told to Reassess Premium Hike for Alzheimer's DrugU.S. health secretary Xavier Becerra is ordering Medicare to reassess a big premium increase facing millions of seniors this year
Medicare Told to Reassess Premium Hike for Alzheimer's DrugU.S. health secretary Xavier Becerra is ordering Medicare to reassess a big premium increase facing millions of seniors this year
Lire la suite »
