The FDA is expected to authorize modified versions of Covid-19 vaccines. The U.S. wants to roll them out before it’s too cold—but that’s before data comes in from human trials
The FDA and vaccine makers say they are confident that shots targeting Omicron subvariants will work safely
With each mutation, the Covid-19 virus is becoming more transmissible. WSJ’s Daniela Hernandez breaks down the science of how Covid variants are getting better at infecting and spreading. Illustration: Rami Abukalamthis week without a staple of its normal decision-making process: data from a study showing whether the shots were safe and worked in humans.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Omicron Boosters Are Coming, But They Weren’t Tested in PeopleThe FDA is considering whether to authorize the first Omicron-specific booster shots, based on animal data
Omicron Boosters Are Coming, But They Weren’t Tested in PeopleThe FDA is considering whether to authorize the first Omicron-specific booster shots, based on animal data
Lire la suite »
 FDA OKs First Targeted Agent, Pemigatinib, for Rare Blood CancerFDA approves pemigatinib, a selective fibroblast growth factor receptor inhibitor, for adults with relapsed or refractory myeloid/lymphoid neoplasms with FGFR1 rearrangement.
FDA OKs First Targeted Agent, Pemigatinib, for Rare Blood CancerFDA approves pemigatinib, a selective fibroblast growth factor receptor inhibitor, for adults with relapsed or refractory myeloid/lymphoid neoplasms with FGFR1 rearrangement.
Lire la suite »
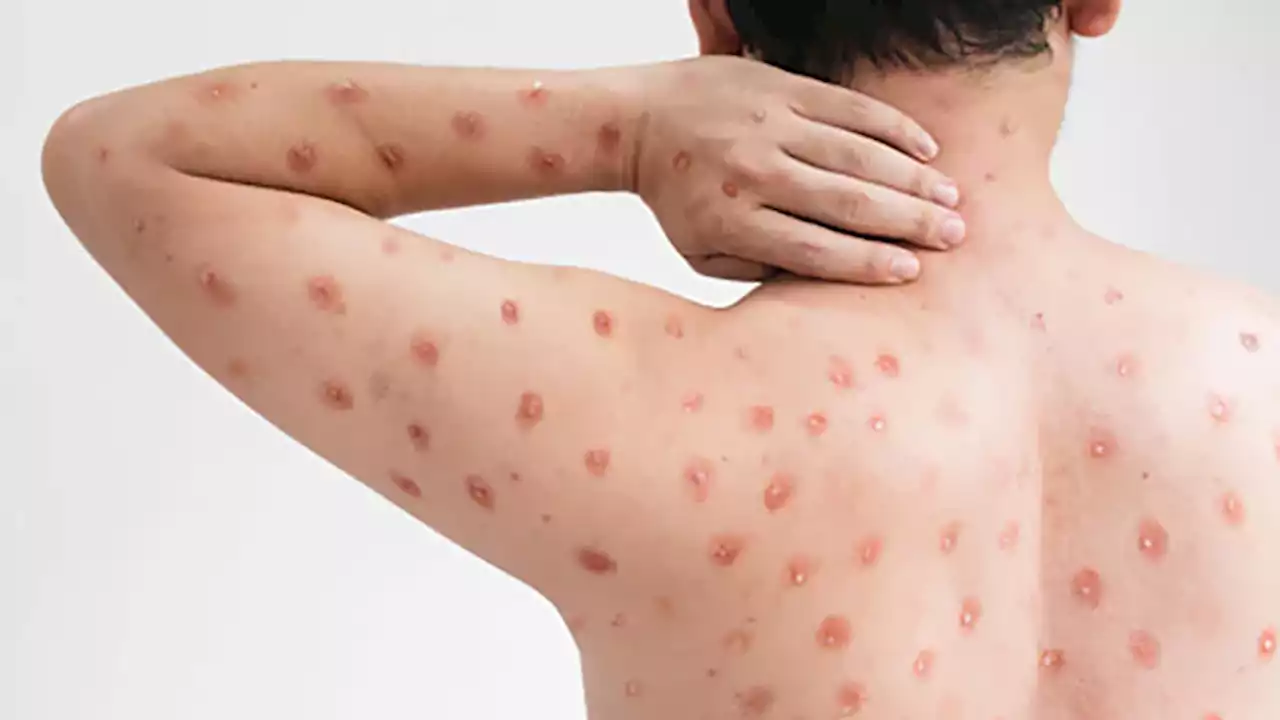 Fecal Transplant Treatments Might Transmit Monkeypox: FDAIt’s not known whether the monkeypox virus can be transmitted through feces, but the agency advised additional safety precautions, including donor screening.
Fecal Transplant Treatments Might Transmit Monkeypox: FDAIt’s not known whether the monkeypox virus can be transmitted through feces, but the agency advised additional safety precautions, including donor screening.
Lire la suite »
![]() Migraine patch developer Zosano Pharma sold in bankruptcy court to Emergex for $1 million - Silicon Valley Business JournalAn East Bay company's 16-year goal to deliver drugs through tiny needles on an adhesive patch comes to an end in bankruptcy court.
Migraine patch developer Zosano Pharma sold in bankruptcy court to Emergex for $1 million - Silicon Valley Business JournalAn East Bay company's 16-year goal to deliver drugs through tiny needles on an adhesive patch comes to an end in bankruptcy court.
Lire la suite »
