The FDA must decide whether to approve the therapy by Dec. 8.
A researcher performs a CRISPR process at the Max Delbrück Center for Molecular Medicine. Photo: Gregor Fischer/dpaEditing genes directly in a patient's body could be life-changing for people with debilitating hereditary disorders like sickle cell disease, which the exa-cel therapy under discussion targets.around "off-target" edits that zero in on the wrong genetic sequence and could increase the risk of developing cancer.
A patient's stem cells are edited to produce high levels of fetal hemoglobin, which can offset the effects of defective hemoglobin produced in patients with sickle cell.didn't take issue with the efficacy of exa-cel but questioned if the companies did sufficient analysis to adequately assess the off-target risk.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
Lire la suite »
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Lire la suite »
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Lire la suite »
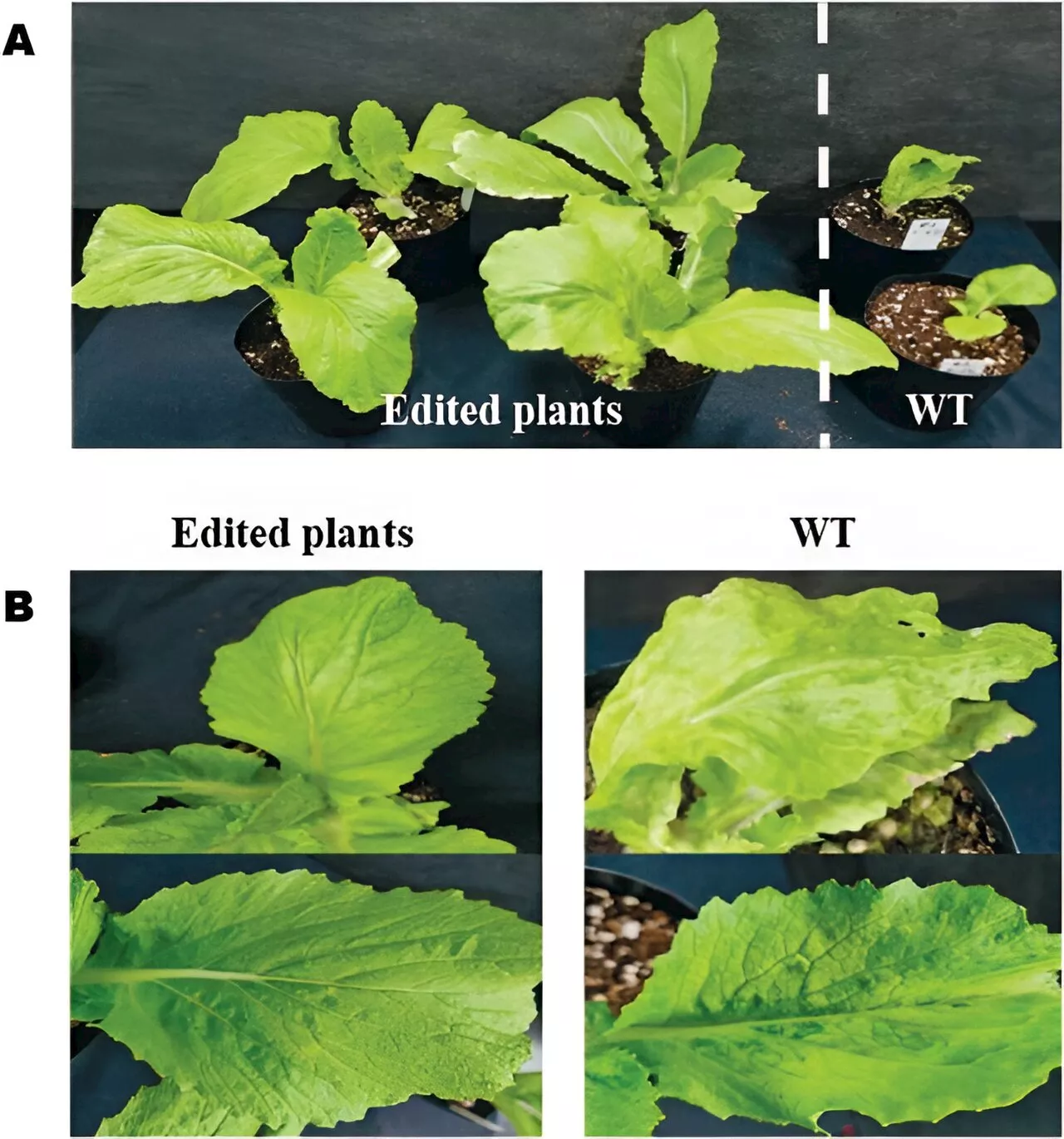 CRISPR/Cas9 unlocks TuMV resistance in Chinese cabbage: A leap forward in genome-edited plant breedingGenome editing, especially the CRISPR/Cas9 technology, holds immense promise in enhancing plant traits, primarily disease resistance, offering a more efficient alternative to traditional breeding.
CRISPR/Cas9 unlocks TuMV resistance in Chinese cabbage: A leap forward in genome-edited plant breedingGenome editing, especially the CRISPR/Cas9 technology, holds immense promise in enhancing plant traits, primarily disease resistance, offering a more efficient alternative to traditional breeding.
Lire la suite »
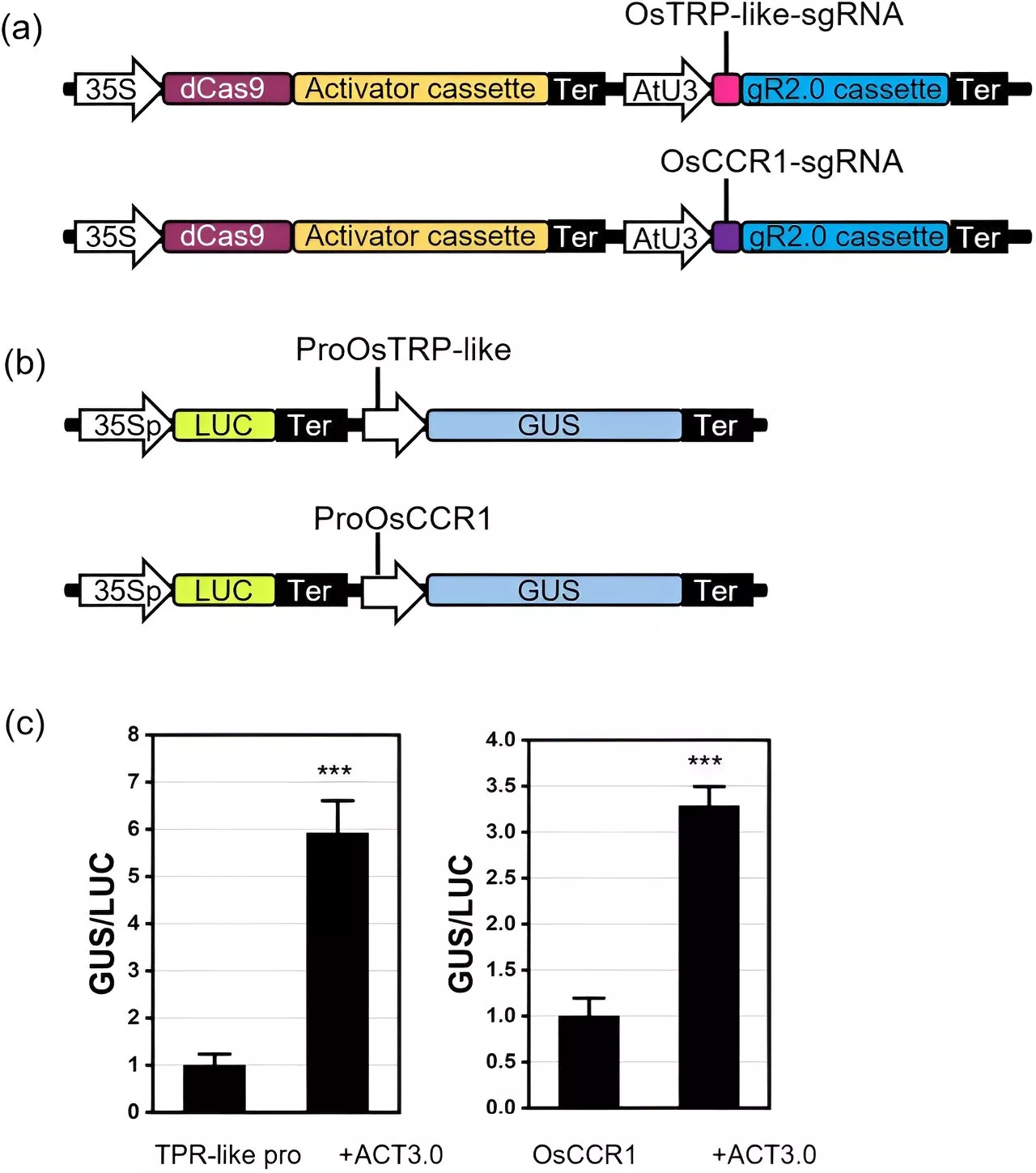 Unlocking the genetic potential of poplars: A new era of precision genome editing with CRISPRThe Populus genus, commonly known as poplars, cottonwoods, and aspens, consists of approximately 30 tree species native to the northern hemisphere. Because of their diverse usages in landscape, agriculture, bioenergy, and industry, Populus species have been the focus of many tree breeding and genetic improvement programs.
Unlocking the genetic potential of poplars: A new era of precision genome editing with CRISPRThe Populus genus, commonly known as poplars, cottonwoods, and aspens, consists of approximately 30 tree species native to the northern hemisphere. Because of their diverse usages in landscape, agriculture, bioenergy, and industry, Populus species have been the focus of many tree breeding and genetic improvement programs.
Lire la suite »
 Potential cure for sickle cell disease raises few concerns for FDA panelVertex Pharmaceuticals has hailed its treatment as 'transformative' for patients with sickle cell disease. The FDA will decide on approval soon.
Potential cure for sickle cell disease raises few concerns for FDA panelVertex Pharmaceuticals has hailed its treatment as 'transformative' for patients with sickle cell disease. The FDA will decide on approval soon.
Lire la suite »
