In the wake of an escalating opioid crisis, members of a joint FDA advisory committee gave the green light to an OTC version of prescription naloxone nasal spray, an opioid overdose reversal agent.
In the wake of an escalating opioid crisis, members of a joint Food and Drug Administration advisory committee gave the green light to an over-the- counter version of prescriptionEvery member of the Nonprescription Drugs Advisory Committee and theand Analgesic Drug Products Advisory Committee agreed that the evidence of benefits from switching the drug to OTC status far outweighs the minimal risks.
Naloxone is an opioid antagonist for the emergency treatment of an opioid overdose. It is available as a prescription in several doses, strengths, dosage forms, and routes of administration. The NARCAN spray delivers a single 4 mg dose intranasally. They stressed the product is easy to administer and does not require inhalation, assembly, or specialized training. They added that it's also easy to carry, store, and is needle-free — so there is no risk of needle-stick injury.
For example, some pharmacies choose not to stock naloxone, prescriptions require access to practitioners familiar with the drug, and current community distribution programs for naloxone are limited.Easier access to naloxone could also reduce the shame and stigma experienced by people who use drugs, experts note.
However, some steps that appeared on the back of the package and on the side panel confused some study participants who started with step three instead of first administering a dose after checking that there had been an overdose. Some noted label improvements, such as having the five steps on a single panel and in a font that facilitates easy reading, can be discussed later — as could the possible need for another HFV study, they said.FDA officials reiterated the goal of the agency to increase access to opioid overdose reversal agents, and to encourage acceleration of the development of nonprescription naloxone.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 U.S. FDA panel backs OTC opioid overdose drug, proposes label changesEmergent BioSolutions Inc's over-the-counter version of opioid overdose reversing drug received unanimous support from U.S. Food and Drug Administration's panel of advisers, sending shares of the contract drugmaker up nearly 16% after market.
U.S. FDA panel backs OTC opioid overdose drug, proposes label changesEmergent BioSolutions Inc's over-the-counter version of opioid overdose reversing drug received unanimous support from U.S. Food and Drug Administration's panel of advisers, sending shares of the contract drugmaker up nearly 16% after market.
Lire la suite »
 FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication
FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication
Lire la suite »
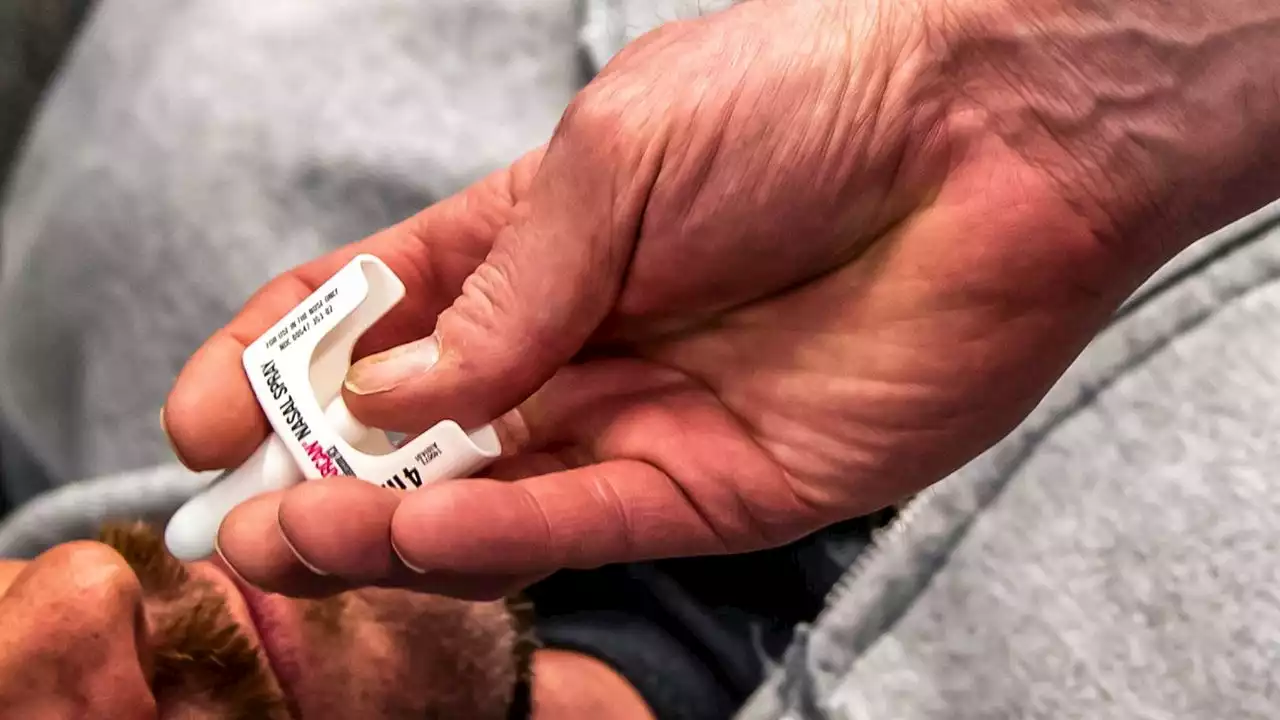 FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone, commonly referred to as Narcan, should be made available as an over-the-counter medication.
FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone, commonly referred to as Narcan, should be made available as an over-the-counter medication.
Lire la suite »
 FDA Panel Backs Moving Opioid Antidote Narcan Over the CounterU.S. health advisers say the overdose-reversal drug naloxone should be made available as an over-the-counter medication. It’s the latest government proposal to increase use of the medication against the opioid overdose crisis. The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations. The positive vote by an Food and Drug Administration advisory panel came despite difficulties understanding how to use the drug in a company study. FDA will make a final decision on the drug in coming weeks.
FDA Panel Backs Moving Opioid Antidote Narcan Over the CounterU.S. health advisers say the overdose-reversal drug naloxone should be made available as an over-the-counter medication. It’s the latest government proposal to increase use of the medication against the opioid overdose crisis. The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations. The positive vote by an Food and Drug Administration advisory panel came despite difficulties understanding how to use the drug in a company study. FDA will make a final decision on the drug in coming weeks.
Lire la suite »
 FDA panel backs moving opioid antidote Narcan over the counterThe nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
FDA panel backs moving opioid antidote Narcan over the counterThe nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
Lire la suite »
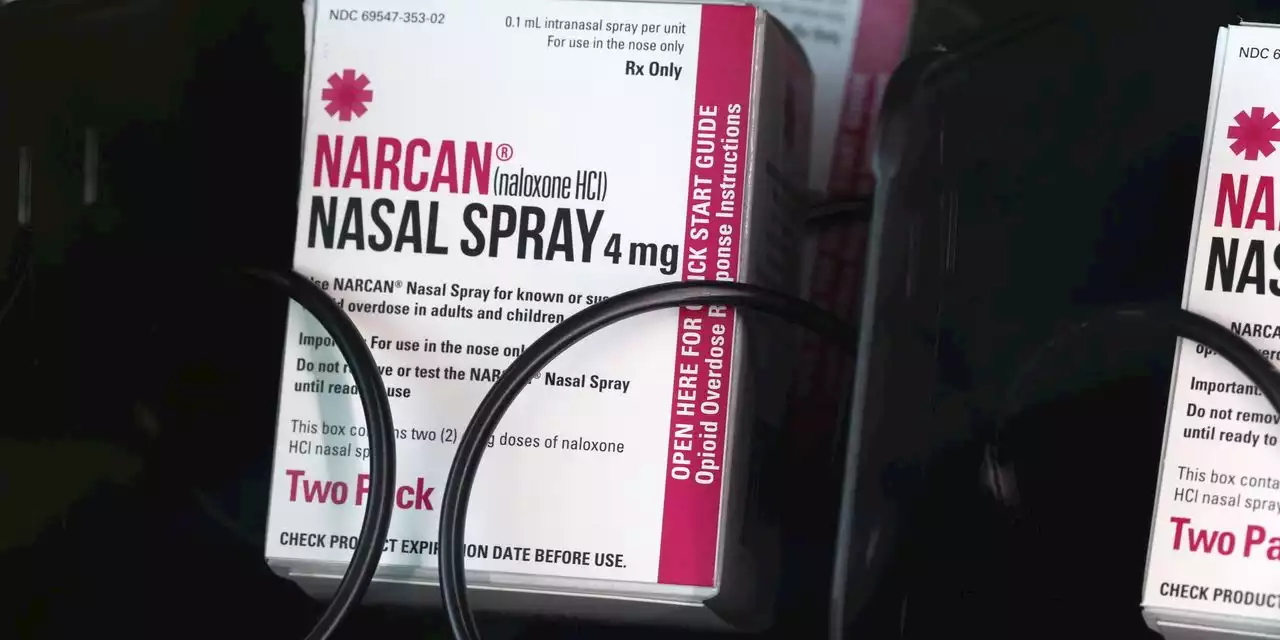 FDA panel says opioid antidote Narcan should be available over the counterThe overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health...
FDA panel says opioid antidote Narcan should be available over the counterThe overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health...
Lire la suite »
