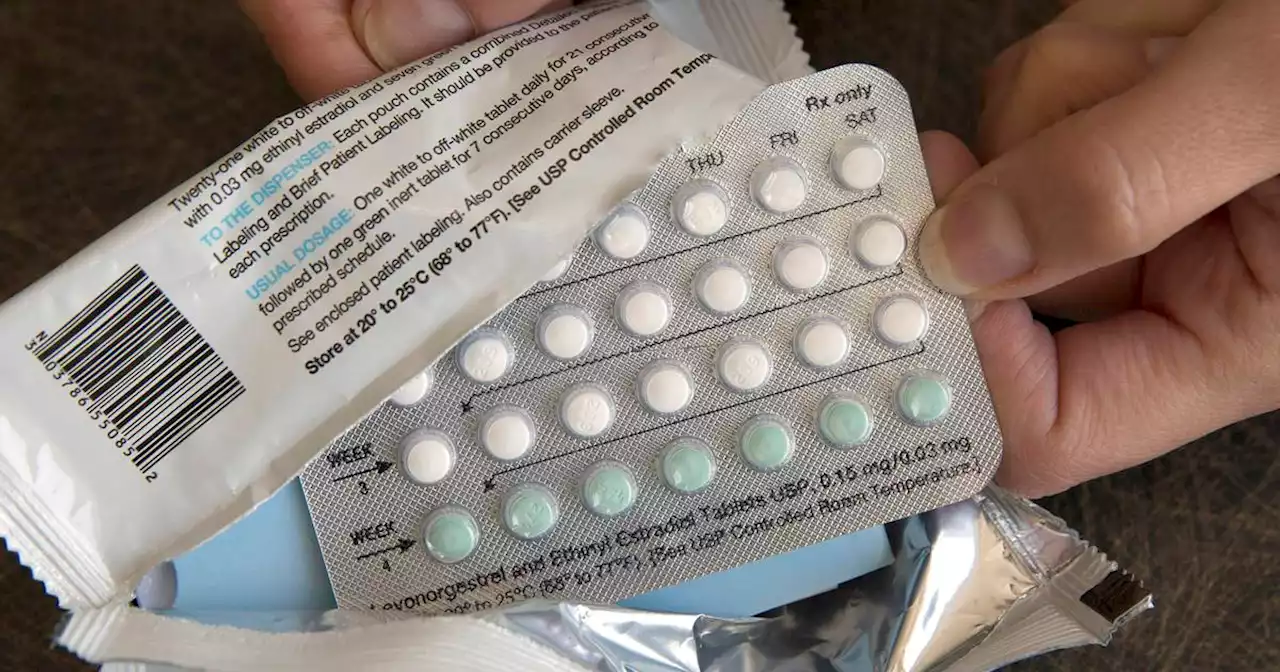
Federal health advisers said that a decades-old birth control pill should be sold without a prescription, paving the way for a likely U.S. approval of the first over-the-counter contraceptive medication.
WASHINGTON — Federal health advisers said Wednesday that a decades-old birth control pill should be sold without a prescription, paving the way for a likely U.S. approval of the first over-the-counter contraceptive medication.
“In the balance between benefit and risk, we’d have a hard time justifying not taking this action,” said Maria Coyle, an Ohio State University pharmacist, who chaired the panel. “The drug is incredibly effective, and I think it will be effective in the over-the-counter realm just as it is in the prescription realm.”
FDA’s decision won’t apply to other birth control pills, only Opill, although advocates hope that an approval decision might push other drugmakers to seek over-the-counter sales. Birth control pills are available without a prescription across much of South America, Asia and Africa. Panel members said almost all women with a history of breast cancer would be under the care of a cancer specialist, who would advise them not to take hormonal drugs that could make their condition worse.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
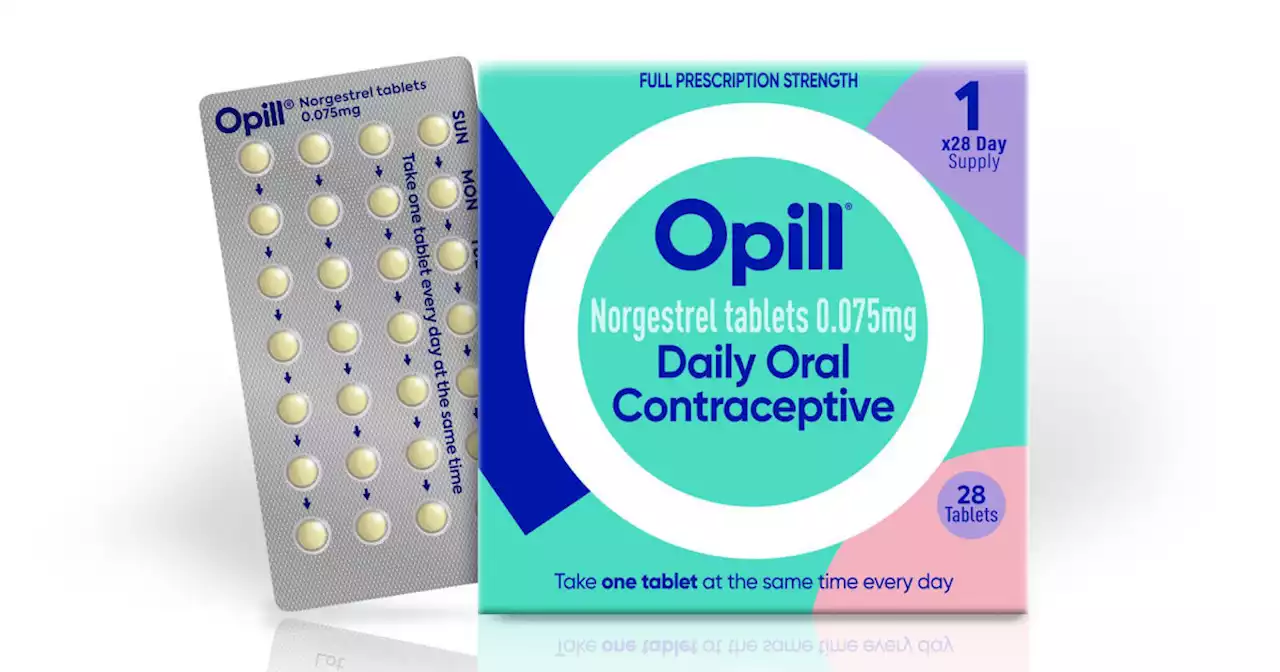 FDA advisory panels back making Opill birth control pill available over-the-counterJUST IN: FDA advisory panels vote unanimously to recommend making a birth control pill available without a prescription. The FDA is expected to decide in late summer whether to follow the recommendation of the committees.
FDA advisory panels back making Opill birth control pill available over-the-counterJUST IN: FDA advisory panels vote unanimously to recommend making a birth control pill available without a prescription. The FDA is expected to decide in late summer whether to follow the recommendation of the committees.
Lire la suite »
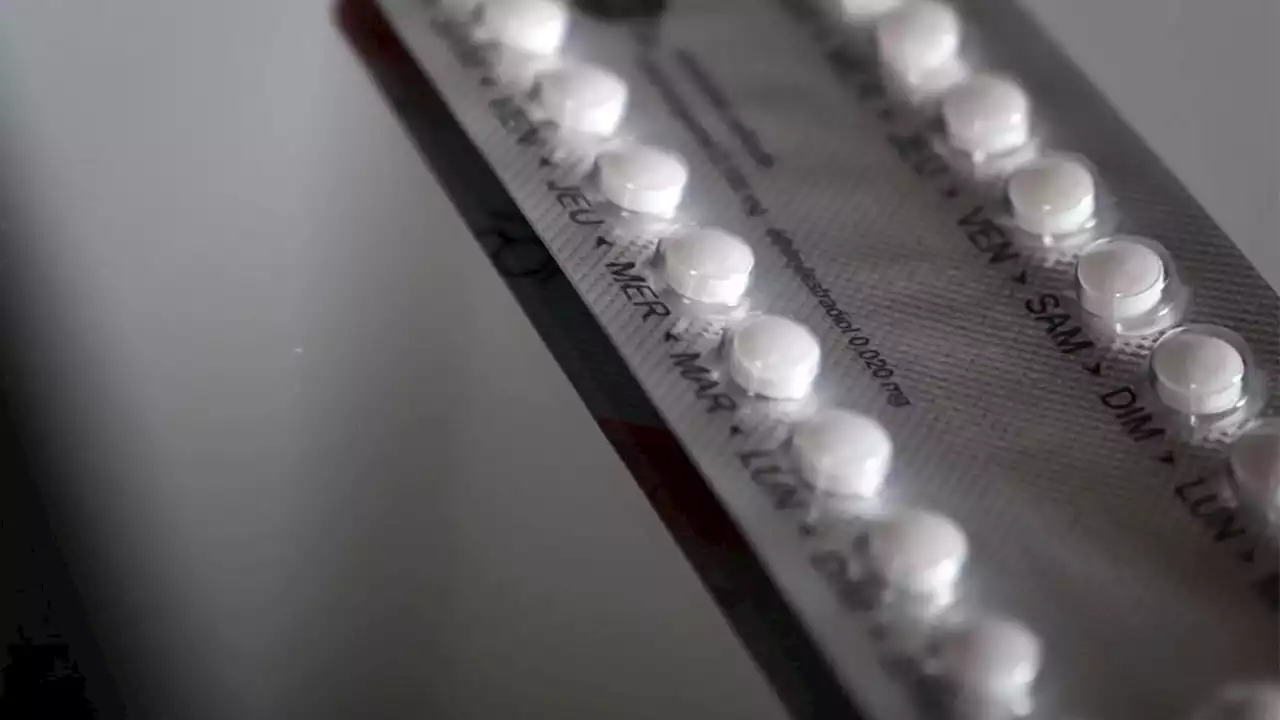 FDA panel backs Opill over-the-counter birth controlFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription.
FDA panel backs Opill over-the-counter birth controlFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription.
Lire la suite »
 FDA panel backs over-the-counter birth control pill, teeing up approvalBREAKING: An FDA panel voted to back the sale of an over-the-counter birth control pill, clearing the way for approval.
FDA panel backs over-the-counter birth control pill, teeing up approvalBREAKING: An FDA panel voted to back the sale of an over-the-counter birth control pill, clearing the way for approval.
Lire la suite »
 FDA advisors recommend sale of birth control pill without a prescriptionFDA scientists raised concerns about whether the general public will understand the drug label and use HRA Pharma's Opill in the right way.
FDA advisors recommend sale of birth control pill without a prescriptionFDA scientists raised concerns about whether the general public will understand the drug label and use HRA Pharma's Opill in the right way.
Lire la suite »
 FDA may allow birth control pill without prescription this summer as experts weigh dataHRA Pharma asked the Food and Drug Administration to allow over-the-counter sales of Opill after the Supreme Court said there was no federal right to abortion.
FDA may allow birth control pill without prescription this summer as experts weigh dataHRA Pharma asked the Food and Drug Administration to allow over-the-counter sales of Opill after the Supreme Court said there was no federal right to abortion.
Lire la suite »
 FDA Experts to Consider First Over-the-Counter Birth Control PillAs a U.S. Food Drug Administration advisory panel prepares to weigh whether to recommend that a birth control pill be sold over the counter in this country, a coalition of advocates on Monday called attention to the safety and effectiveness of the medication.
FDA Experts to Consider First Over-the-Counter Birth Control PillAs a U.S. Food Drug Administration advisory panel prepares to weigh whether to recommend that a birth control pill be sold over the counter in this country, a coalition of advocates on Monday called attention to the safety and effectiveness of the medication.
Lire la suite »