The governmental agency said it has “conclusive scientific evidence” to back disapproval of the citrus-boosting additive brominated vegetable oil (BVO).
The Food and Drug Administration"proposed to revoke the regulation authorizing the use of brominated vegetable oil in food," claiming the ingredient"is no longer considered safe."10 foods that need to be refrigerated — but not everyone does
The FDA announced that the toxicology reports have given the administration “conclusive scientific evidence to support proposal to remove the FDA’s food additive authorization for BVO.” “It’s also been linked to neurologic symptoms in people who drink large quantities of citrus soda — more than 2 liters a day.”
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
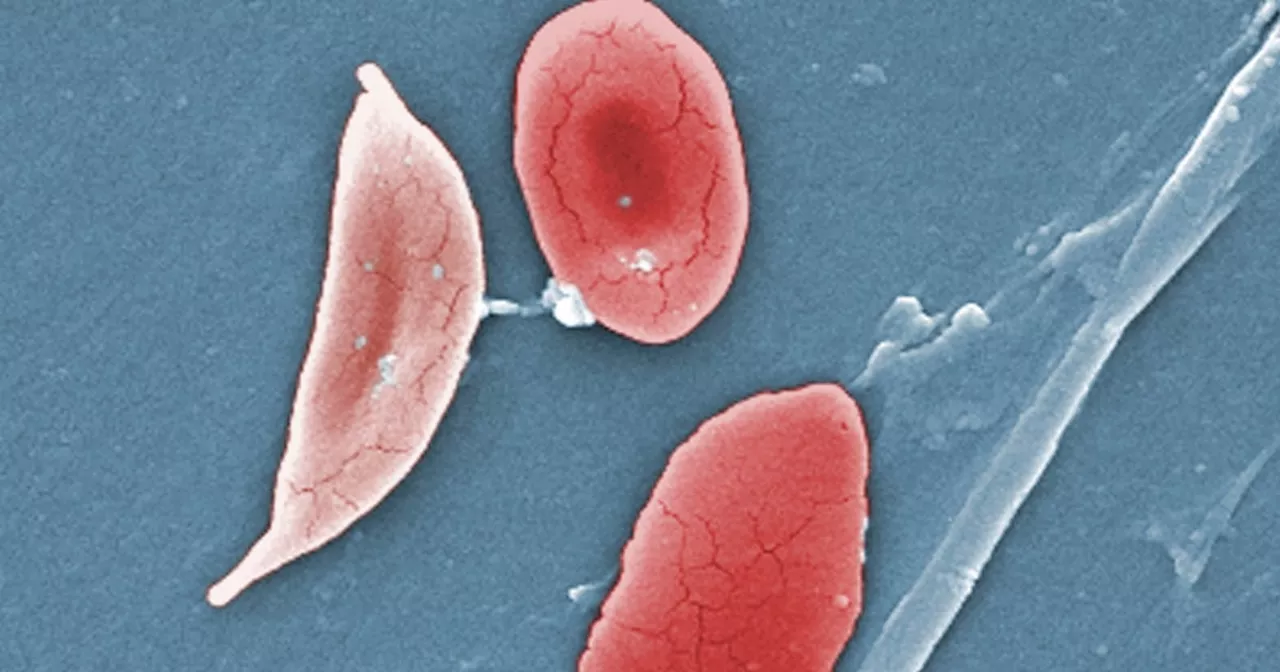 FDA moves closer to sickle cell cure that uses gene editingBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA moves closer to sickle cell cure that uses gene editingBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Lire la suite »
 Potential cure for sickle cell disease raises few concerns for FDA panelVertex Pharmaceuticals has hailed its treatment as 'transformative' for patients with sickle cell disease. The FDA will decide on approval soon.
Potential cure for sickle cell disease raises few concerns for FDA panelVertex Pharmaceuticals has hailed its treatment as 'transformative' for patients with sickle cell disease. The FDA will decide on approval soon.
Lire la suite »
 U.S. FDA approves Amgen's biosimilar version of J&J's psoriasis drugU.S. FDA approves Amgen's biosimilar version of J&J's psoriasis drug
U.S. FDA approves Amgen's biosimilar version of J&J's psoriasis drugU.S. FDA approves Amgen's biosimilar version of J&J's psoriasis drug
Lire la suite »
 Amgen gets FDA approval for its inflammatory diseases treatment WezlanaAmgen has received approval from the U.S. Food and Drug Administration for Wezlana, its treatment for multiple inflammatory diseases.
Amgen gets FDA approval for its inflammatory diseases treatment WezlanaAmgen has received approval from the U.S. Food and Drug Administration for Wezlana, its treatment for multiple inflammatory diseases.
Lire la suite »
 FDA Approves Secukinumab for Adults With Hidradenitis SuppurativaThis marks the second FDA-approved agent for the condition, and the first IL-17A inhibitor.
FDA Approves Secukinumab for Adults With Hidradenitis SuppurativaThis marks the second FDA-approved agent for the condition, and the first IL-17A inhibitor.
Lire la suite »
 FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
Lire la suite »
