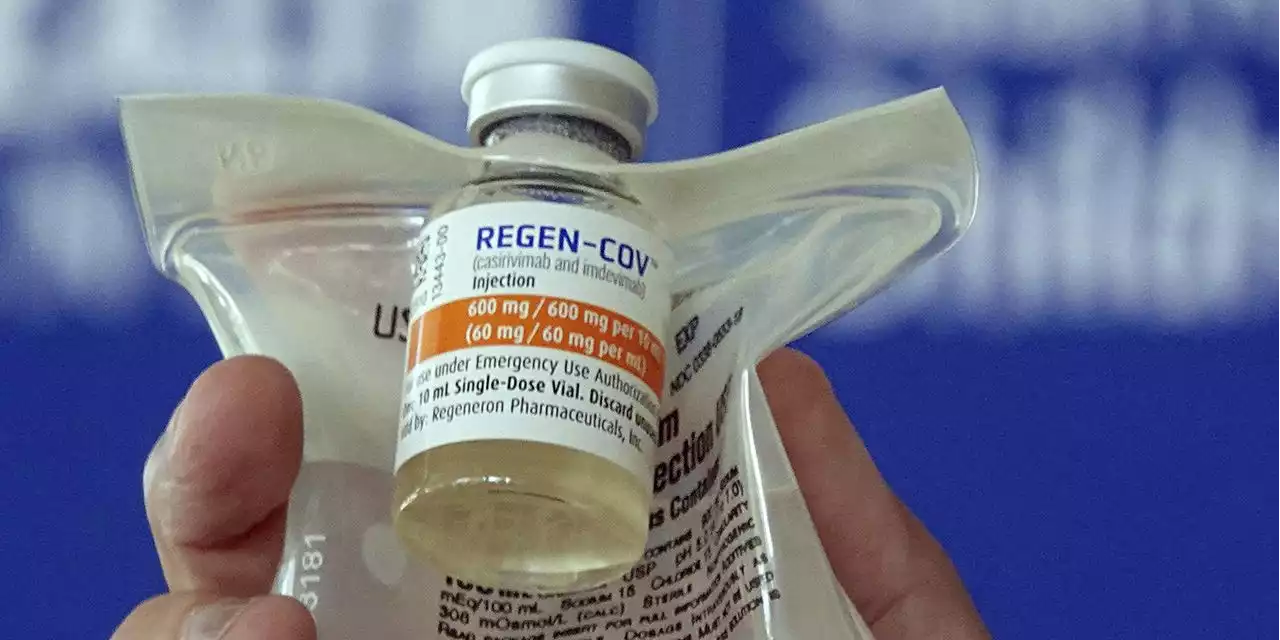
COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant. Doctors have alternate therapies, including two new antiviral pills from Pfizer and Merck, but both are in short supply.
WASHINGTON — COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday.
Omicron’s resistance to the two leading monoclonal antibody medicines has upended the treatment playbook for COVID-19 in recent weeks. The FDA noted in its decision that omicron accounts for more than 99% of U.S. infections, making it “highly unlikely” the antibodies would help people now seeking treatment. The agency said restricting their use would also eliminate unnecessary drug side effects, including allergic reactions.
The drugs are not a substitute for vaccination and are generally reserved for people who are the most vulnerable, including seniors, transplant recipients and those with conditions like heart disease and diabetes. The move comes days after regulators broadened the use of remdesivir — the first drug approved for COVID-19 — to treat more patients.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Biden Administration Limits Use of Regeneron and Lilly Covid-19 Antibody DrugsThe FDA revised the authorizations for the therapies, which don’t work against the Omicron variant, to make sure doctors only use them on non-Omicron patients, and the Health and Human Services Department stopped shipments.
Biden Administration Limits Use of Regeneron and Lilly Covid-19 Antibody DrugsThe FDA revised the authorizations for the therapies, which don’t work against the Omicron variant, to make sure doctors only use them on non-Omicron patients, and the Health and Human Services Department stopped shipments.
Lire la suite »
 Covid-19 news: Strain on health services led to extra non-covid deathsToday’s covid-19 news: · 4000 excess non-covid 19 deaths occurred in England during first year of pandemic · Acute phase of pandemic could end in 2022 with improved vaccination rates, says WHO · Israel recommends fourth vaccine dose for all adults
Covid-19 news: Strain on health services led to extra non-covid deathsToday’s covid-19 news: · 4000 excess non-covid 19 deaths occurred in England during first year of pandemic · Acute phase of pandemic could end in 2022 with improved vaccination rates, says WHO · Israel recommends fourth vaccine dose for all adults
Lire la suite »
 FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
Lire la suite »
 No toboggan slide at Eagles Mere again this year due to COVID-19, lack of iceThis is the ninth straight year the slide, a major fundraiser for volunteer fire companies in Sullivan County, has not been built.
No toboggan slide at Eagles Mere again this year due to COVID-19, lack of iceThis is the ninth straight year the slide, a major fundraiser for volunteer fire companies in Sullivan County, has not been built.
Lire la suite »
 LA County Sees Drop In COVID-19 Hospitalizations, But Transmission Rate Remains HighThe winter surge of coronavirus cases seems to be tapering slightly though medical professionals caution the public to maintain precautions.
LA County Sees Drop In COVID-19 Hospitalizations, But Transmission Rate Remains HighThe winter surge of coronavirus cases seems to be tapering slightly though medical professionals caution the public to maintain precautions.
Lire la suite »