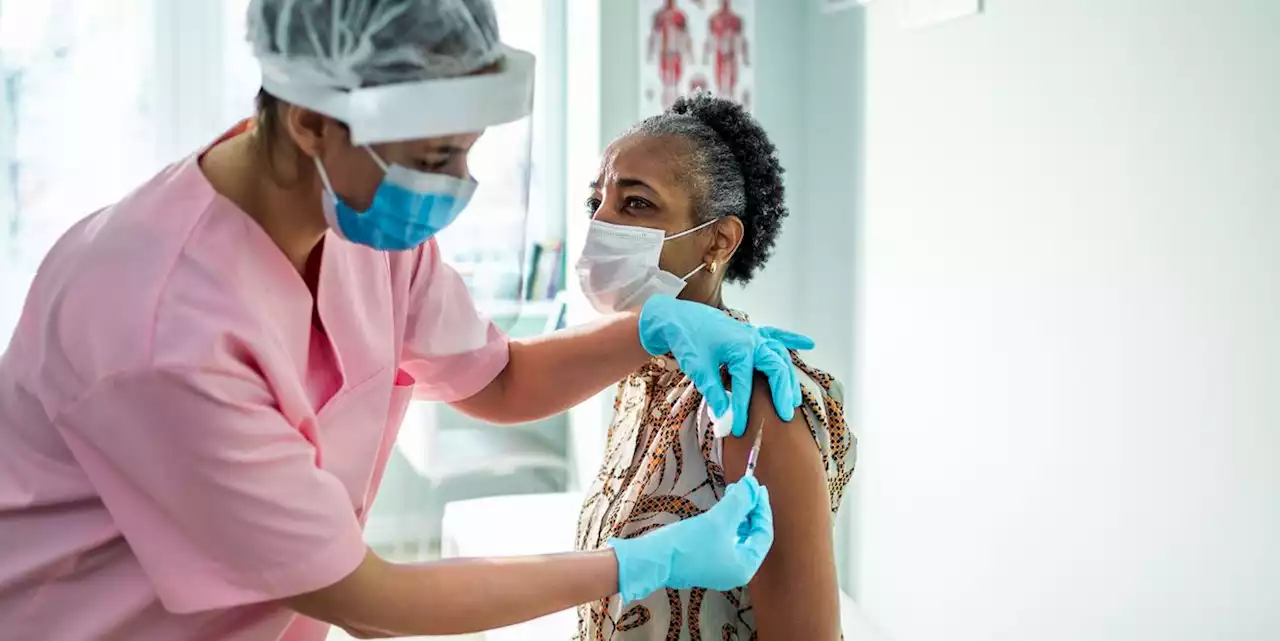
FDA Authorizes a Second COVID-19 Booster Shot for Adults Over 50
“Initial booster dose is critical in helping to protect all adults.”
“Current evidence suggests some waning of protection over time against serious outcomes from COVID-19 in older and immunocompromised individuals. Based on an analysis of emerging data, a second booster dose of either the Pfizer-BioNTech or Moderna COVID-19 vaccine could help increase protection levels for these higher-risk individuals,”, director of the FDA’s Center for Biologics Evaluation and Research said in the FDA press release.
Anyone over the age of 50 years old who has already received their first three vaccine doses more than four months ago can now get a second booster dose of the Pfizer-BioNTech COVID-19 vaccine or Moderna COVID-19 vaccine.from a medical condition, organ transplants, or other illness, who have already received their first three vaccine doses more than four months ago are eligible to receive a second booster dose of the Pfizer-BioNTech COVID-19 vaccine.
Those 18 years or older who are immunocompromised and received their third COVID-19 vaccine more than four months are eligible for a second booster dose of Moderna COVID-19 vaccine. Anyone who received a Johnson & Johnson vaccine and booster dose at least four months ago may now receive a second booster shot from Moderna or Pfizer-BioNTech, according to aAt this time, the booster recommendations for other individuals have not changed. The CDC continues to recommend all eligible adults and children five years and older get their COVID-19 vaccines and a booster when eligible.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
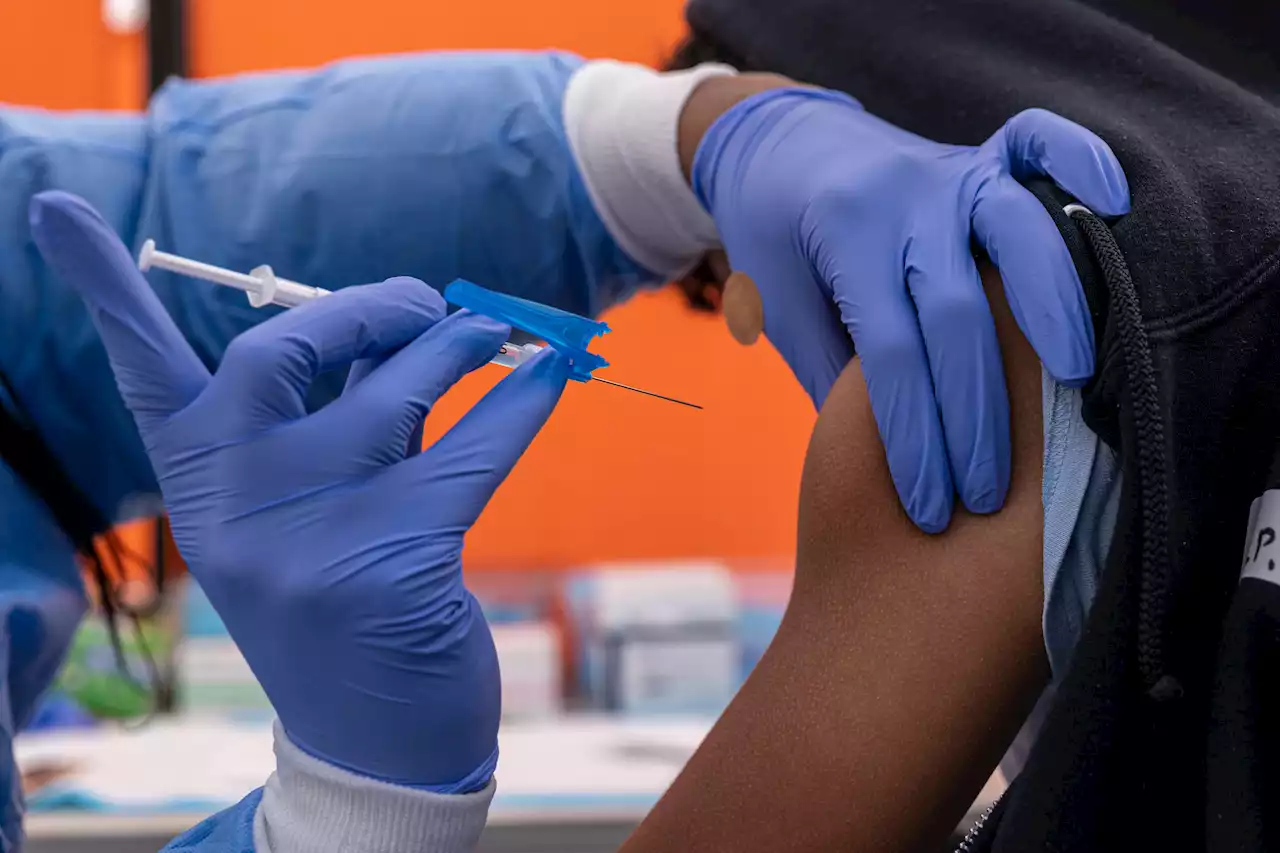 FDA Authorizes Fourth Pfizer and Moderna Covid Vaccine Doses for People Age 50 and OlderThe FDA made the decision comes without consulting its committee of independent vaccine experts.
FDA Authorizes Fourth Pfizer and Moderna Covid Vaccine Doses for People Age 50 and OlderThe FDA made the decision comes without consulting its committee of independent vaccine experts.
Lire la suite »
 U.S. FDA authorizes second COVID booster dose for people aged 50 and olderThe U.S. Food and Drug Administration said Tuesday it has authorized a second booster dose of either the Pfizer/BioNTech undefinedundefined or Moderna...
U.S. FDA authorizes second COVID booster dose for people aged 50 and olderThe U.S. Food and Drug Administration said Tuesday it has authorized a second booster dose of either the Pfizer/BioNTech undefinedundefined or Moderna...
Lire la suite »
FDA authorizes second booster of COVID vaccine for ages 50 and overBREAKING: The FDA authorized second booster shots of Pfizer and Moderna's COVID vaccines for Americans age 50 and older.
Lire la suite »
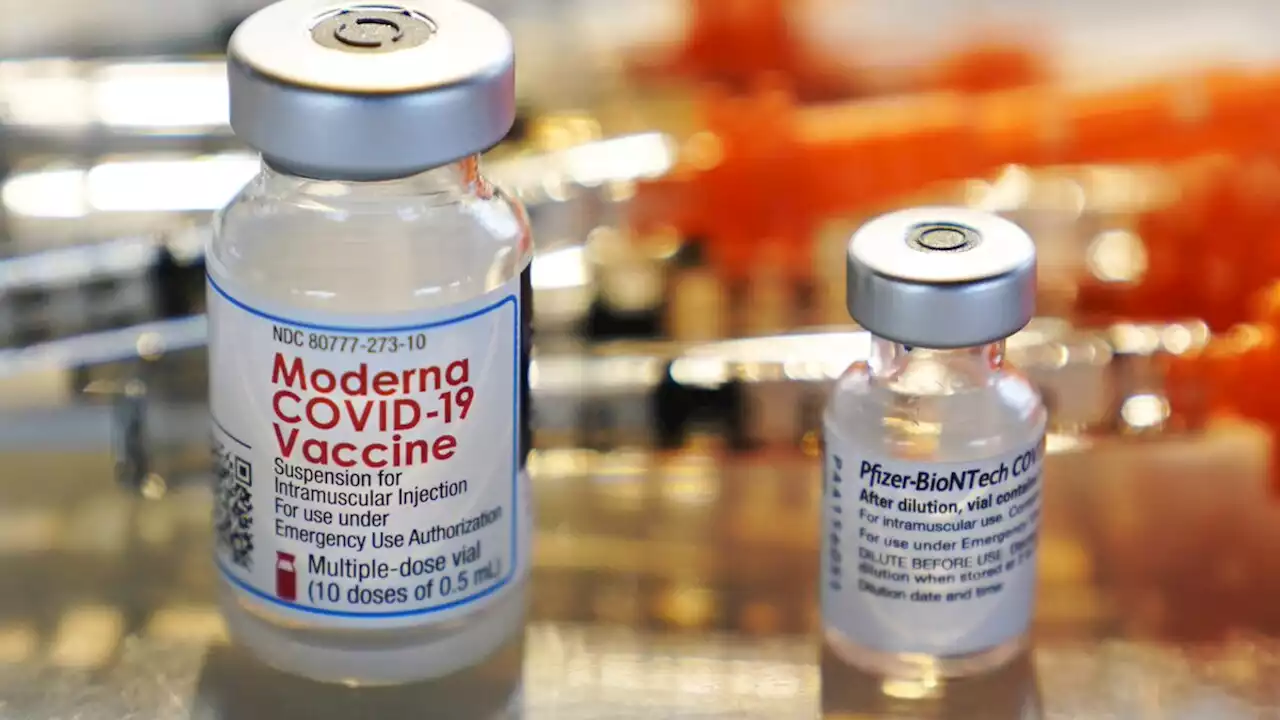 FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
Lire la suite »
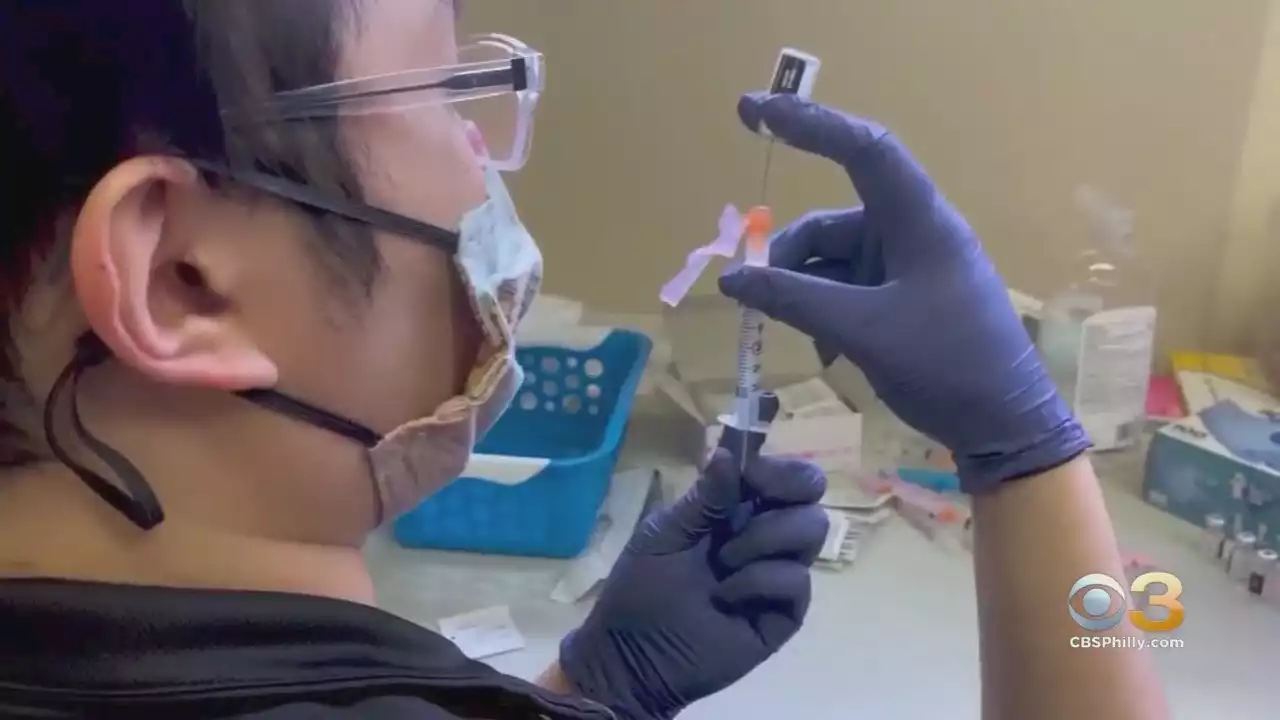 FDA Authorizes Second COVID-19 Booster Shots For Adults Age 50 And OlderJUST IN: The US_FDA has expanded the emergency use authorization of the Pfizer and Moderna COVID-19 vaccines to allow adults age 50 and older to get a second booster as early as 4 months after their first booster dose of any COVID-19 vaccine
FDA Authorizes Second COVID-19 Booster Shots For Adults Age 50 And OlderJUST IN: The US_FDA has expanded the emergency use authorization of the Pfizer and Moderna COVID-19 vaccines to allow adults age 50 and older to get a second booster as early as 4 months after their first booster dose of any COVID-19 vaccine
Lire la suite »