The U.S. Food and Drug Administration (FDA) on Thursday approved the world's first respiratory syncytial virus (RSV) vaccine for older adults. via JessicaDenver7
AREXVY, produced by biopharma company GSK, is a single-shot vaccine for adults 60-years-old and older.“Very exciting. I don't think people realize how often that individuals get sick from RSV,” said Dr. Michelle Barron with UC Health.
According to the Centers for Disease Control and Prevention , roughly 160,000 older adults are hospitalized with RSV every year, and between 6,000 and 10,000 die. “They can have exacerbations of underlying lung issues — some people just really can't breathe. And it's not unlike what happens to kids, where you start to have a lot of wheezing and coughing and you just can't breathe,” Barron said.There’s no specific treatment for the virus.
RSV is typically associated with severe illness in young children. Spikes in the virus lead to hundreds of hospitalizations of children this past fall in the Denver metro area.“There’s also a study that was recently published looking at women that were pregnant and giving it to them as a way of preventing getting the baby sick, because then you give the baby some of the antibody and also mom doesn't get sick,” Barron said.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA approves world's first RSV vaccine, a shot for adults ages 60 and upBREAKING: FDA approves the world's first respiratory syncytial virus, or RSV, vaccine made by pharmaceutical giant GSK.
FDA approves world's first RSV vaccine, a shot for adults ages 60 and upBREAKING: FDA approves the world's first respiratory syncytial virus, or RSV, vaccine made by pharmaceutical giant GSK.
Lire la suite »
FDA approves world’s first RSV vaccine, a shot for older adultsIn a late-stage clinical trial, the single-dose shot lowered the risk of symptomatic illness by 83% and of severe illness by 94%.
Lire la suite »
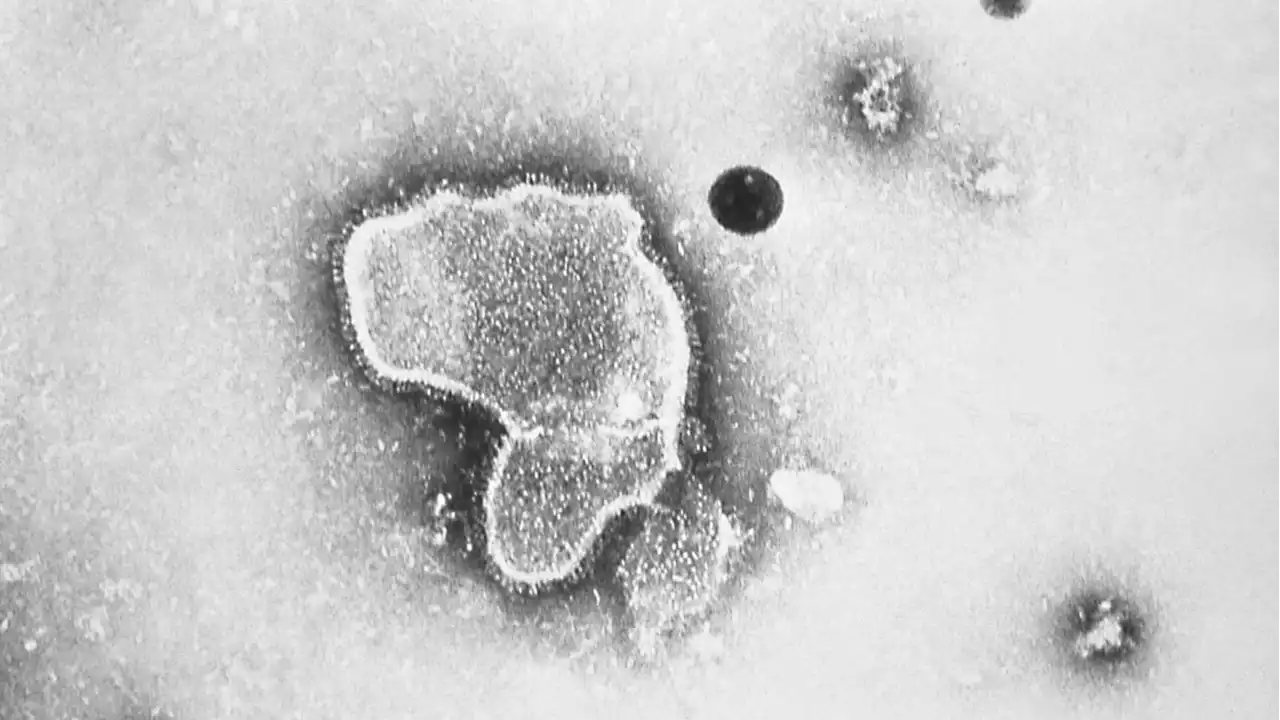 FDA Approves World’s First RSV Vaccine for Adults 60 and OlderResearchers first began attempting to develop a vaccine for respiratory syncytial virus, which is of particular danger to older people and infants, around 60 years ago.
FDA Approves World’s First RSV Vaccine for Adults 60 and OlderResearchers first began attempting to develop a vaccine for respiratory syncytial virus, which is of particular danger to older people and infants, around 60 years ago.
Lire la suite »
 World’s first RSV vaccine approved by FDA — breakthrough 60 years in the making“Our focus now is to ensure eligible older adults in the US can access the vaccine as quickly as possible,” said Tony Wood, the chief scientific officer at GSK.
World’s first RSV vaccine approved by FDA — breakthrough 60 years in the making“Our focus now is to ensure eligible older adults in the US can access the vaccine as quickly as possible,” said Tony Wood, the chief scientific officer at GSK.
Lire la suite »
 FDA approves GSK's RSV vaccine for older adults, world's first shot against virusThe FDA's approval is a victory for GSK in a race against Pfizer and Moderna to bring a shot that targets respiratory syncytial virus to the market.
FDA approves GSK's RSV vaccine for older adults, world's first shot against virusThe FDA's approval is a victory for GSK in a race against Pfizer and Moderna to bring a shot that targets respiratory syncytial virus to the market.
Lire la suite »
