GSK's Arexvy, the first RSV vaccine approved for use in the U.S., gets the green light for older adults.
The Food and Drug Administration on Wednesday approved GSK’s GSK vaccine for respiratory syncytial virus , a highly contagious virus that causes lung and breathing-passage infections.
“Older... The Food and Drug Administration on Wednesday approved GSK’s GSK vaccine for respiratory syncytial virus , a highly contagious virus that causes lung and breathing-passage infections. “Older adults, in particular those with underlying health conditions, such as heart or lung disease or weakened immune systems, are at high risk for severe disease caused by RSV,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. Among people 65 and older, RSV leads to about 60,000 to 120,000 hospitalizations and 6,000 to 10,000 deaths each year, according to the Centers for Disease Control and Prevention.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 FDA approves world's first RSV vaccine, a shot for adults ages 60 and upBREAKING: FDA approves the world's first respiratory syncytial virus, or RSV, vaccine made by pharmaceutical giant GSK.
FDA approves world's first RSV vaccine, a shot for adults ages 60 and upBREAKING: FDA approves the world's first respiratory syncytial virus, or RSV, vaccine made by pharmaceutical giant GSK.
Lire la suite »
 FDA approves first vaccine for RSV, a moment six decades in the making | CNNAfter a 60-year scientific quest, the world has its first vaccine to protect against respiratory syncytial virus, or RSV -- and more are on the way.
FDA approves first vaccine for RSV, a moment six decades in the making | CNNAfter a 60-year scientific quest, the world has its first vaccine to protect against respiratory syncytial virus, or RSV -- and more are on the way.
Lire la suite »
RSV in First Year Could Raise Baby’s Risk of AsthmaChildren who didn’t contract RSV in their first year of life had a 26 percent lower risk of asthma later in life. Learn more about the connection here.
Lire la suite »
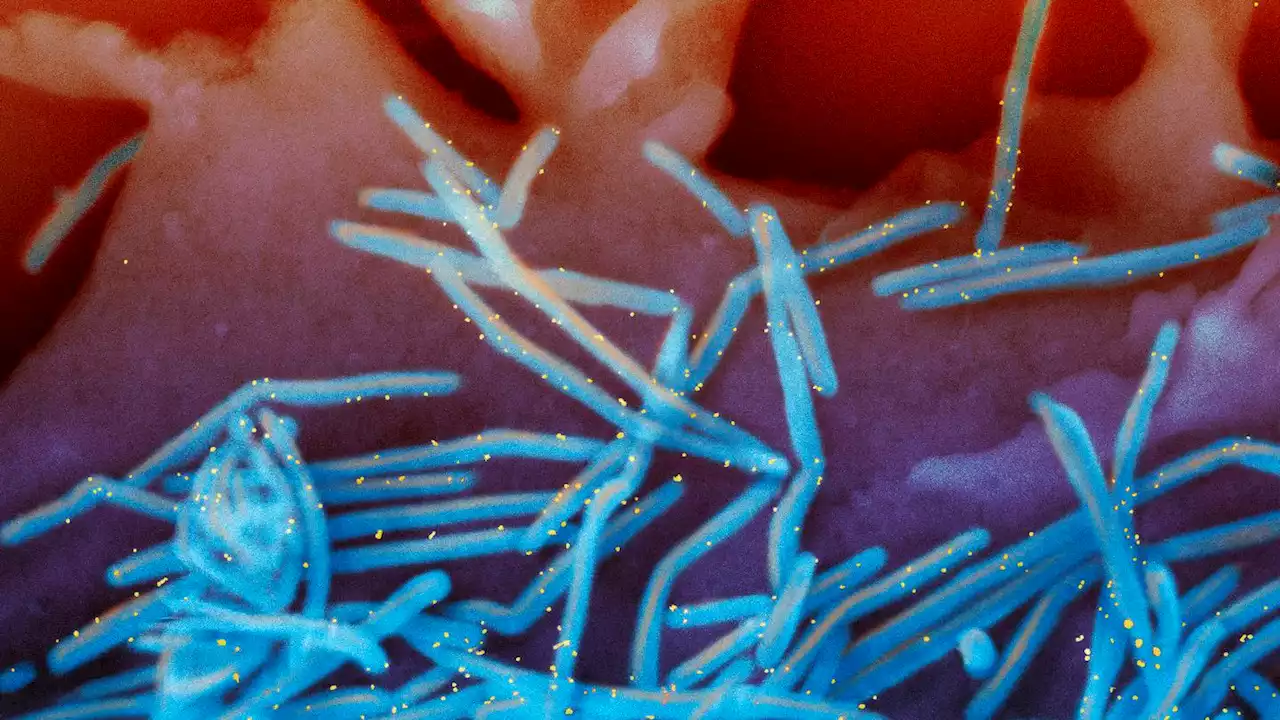 US approves 1st vaccine for RSV after decades of attemptsThe U.S. has approved the first vaccine for RSV, shots to protect older adults
US approves 1st vaccine for RSV after decades of attemptsThe U.S. has approved the first vaccine for RSV, shots to protect older adults
Lire la suite »
