The pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled
FILE - Medical staff prepares an AstraZeneca coronavirus vaccine during preparations at the vaccine center in Ebersberg near Munich, Germany, Monday, March 22, 2021. The pharma giant AstraZeneca has requested that its European authorization for its COVID vaccine be pulled, according to the EU medicines regulator on Wednesday, May 8, 2024. , according to the EU medicines regulator.
AstraZeneca's COVID-19 vaccine was first given the nod by the EMA in January 2021. Within weeks, however, concerns grew about the vaccine's safety, when dozens of countries suspended the vaccine's use after unusual but rare blood clots were detected in a small number of immunized people. The EU regulator concluded AstraZeneca's shot didn't raise the overall risk of clots, but doubts remained.
Business Medication COVID-19 Pandemic Immunizations World News General News Article 110020136
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled. In an update on the European Medicines Agency’s website Wednesday, the regulator said that the approval for AstraZeneca’s Vaxzevria had been withdrawn at the company's request.
European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled. In an update on the European Medicines Agency’s website Wednesday, the regulator said that the approval for AstraZeneca’s Vaxzevria had been withdrawn at the company's request.
Lire la suite »
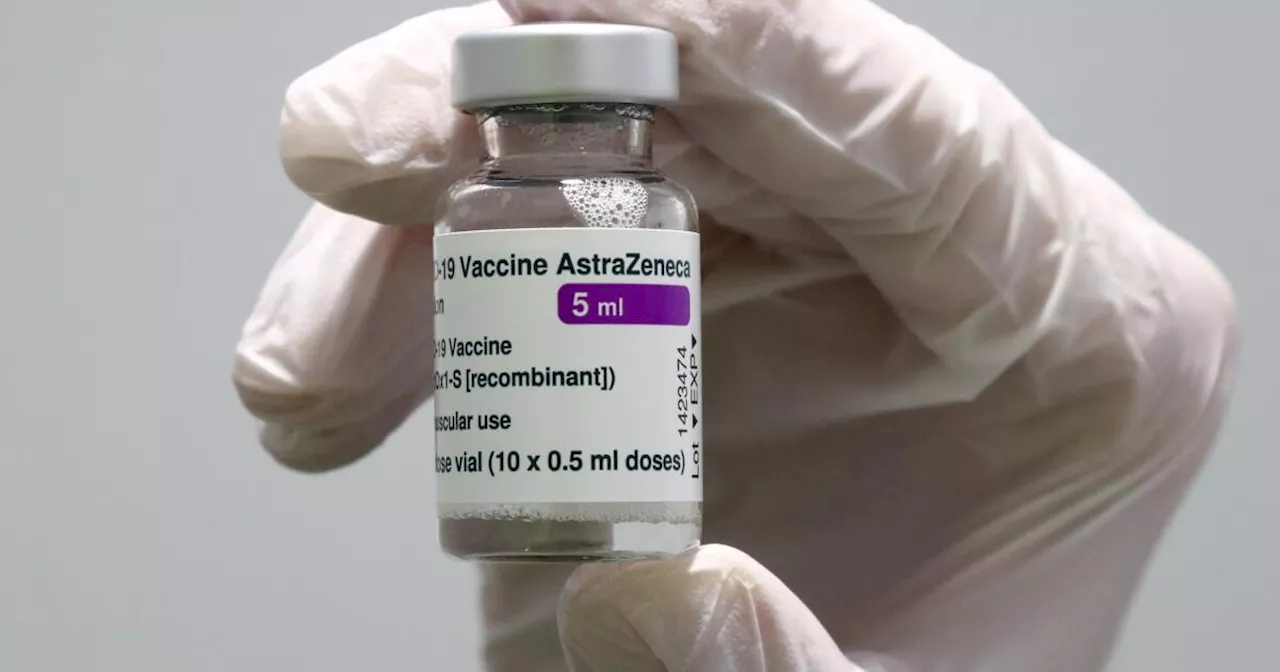 European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled
European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled
Lire la suite »
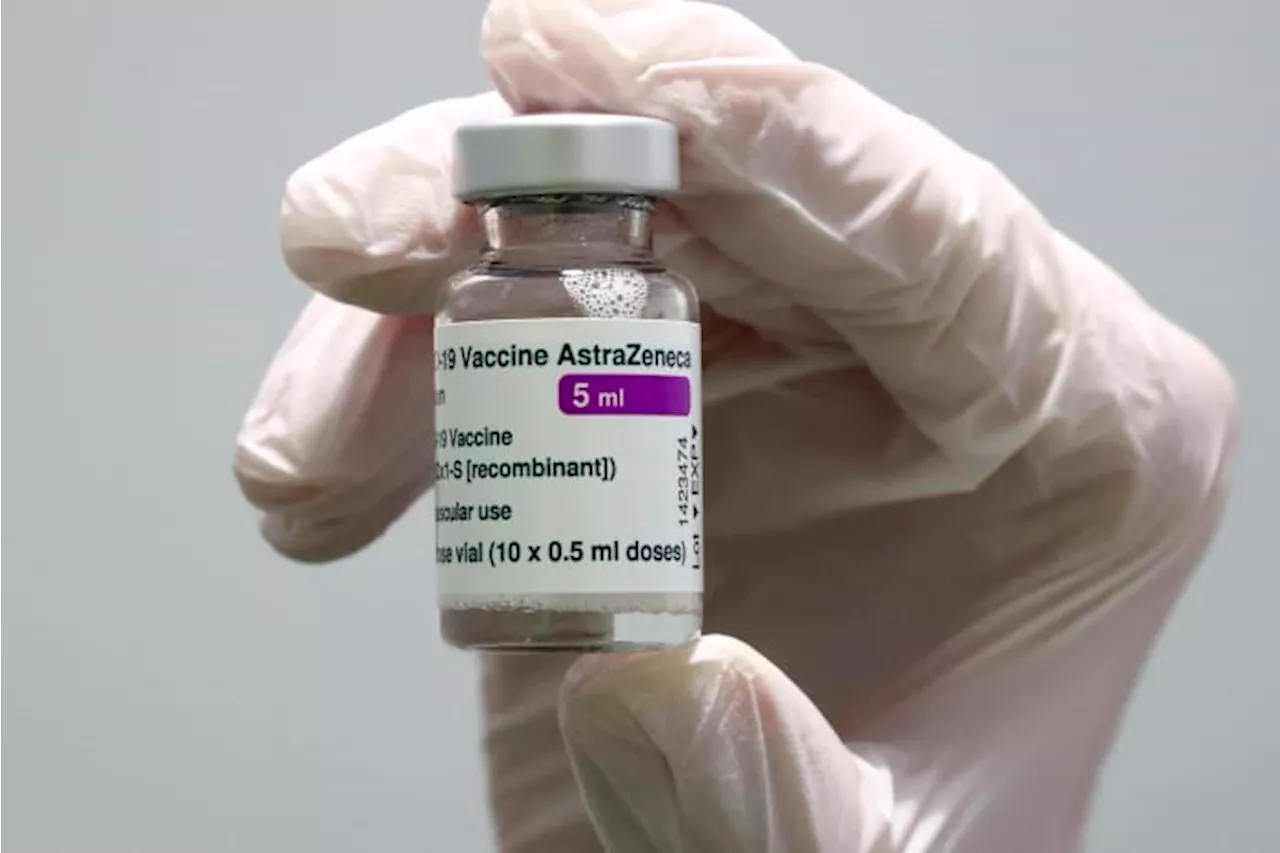 European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled.
European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled.
Lire la suite »
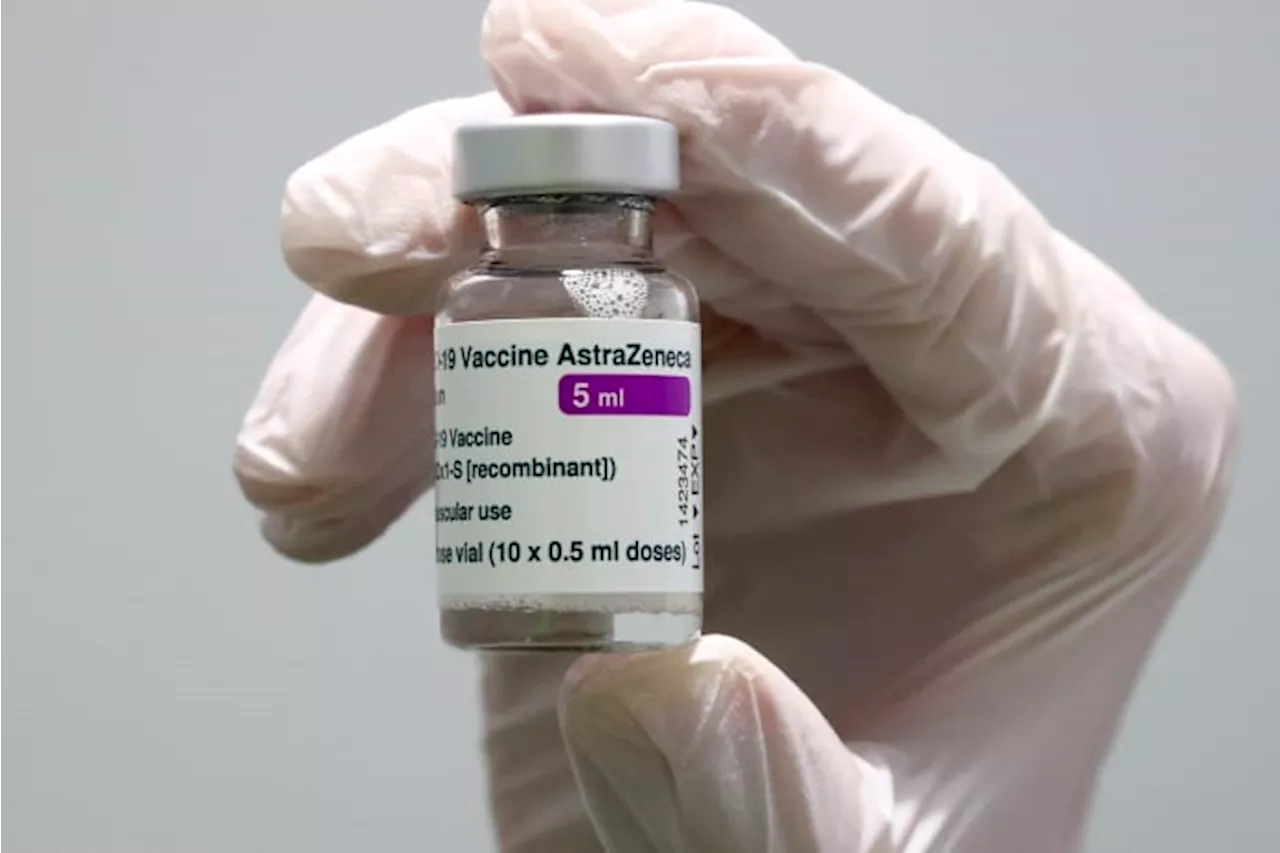 European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled.
European Medicines Agency pulls authorization for AstraZeneca's COVID shot, at company's requestThe pharma giant AstraZeneca has requested that the European authorization for its COVID-19 vaccine be pulled.
Lire la suite »
 AstraZeneca withdraws COVID-19 vaccine from worldwide circulationAstraZeneca is withdrawing its COVID-19 vaccine from worldwide circulation and marketing authorizations.
AstraZeneca withdraws COVID-19 vaccine from worldwide circulationAstraZeneca is withdrawing its COVID-19 vaccine from worldwide circulation and marketing authorizations.
Lire la suite »
 AstraZeneca admits its COVID vaccine can cause deadly blood clotsAstraZeneca has revealed that its COVID-19 vaccine can lead to a condition known as thrombosis with thrombocytopenia syndrome, which can be fatal.
AstraZeneca admits its COVID vaccine can cause deadly blood clotsAstraZeneca has revealed that its COVID-19 vaccine can lead to a condition known as thrombosis with thrombocytopenia syndrome, which can be fatal.
Lire la suite »
