Analysts are bullish on the prospects for exa-cel, which would be the first approved drug using the gene-editing tech called Crispr
Crispr Therapeutics AG’s stock rallied 12% Wednesday, after a positive meeting of a Food and Drug Administration advisory panel on a treatment for sickle-cell disease raised hopes it will win regulatory approval.
Sickle-cell disease is a painful inherited blood disorder that affects an estimated 70,000 to 100,000 Americans a year, according to the American Society of Hematology. In this case, the panel reviewed the drug’s potential off-target effects. A drug used to treat one particular condition or symptom can have effects on other pathways or tissues within the body in addition to the intended target, according to the National Institutes of Health. These are known as off-target effects, and are tracked because they can lead to side effects ranging in severity from mild to life-threatening.
Truist analysts said the event felt like a check-the-box and said they expect approval by, or even before, Dec. 8.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Crispr Therapeutics’ stock soars premarket after positive meeting of FDA panel on sickle-cell disease therapyCiara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
Crispr Therapeutics’ stock soars premarket after positive meeting of FDA panel on sickle-cell disease therapyCiara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
Lire la suite »
 FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
Lire la suite »
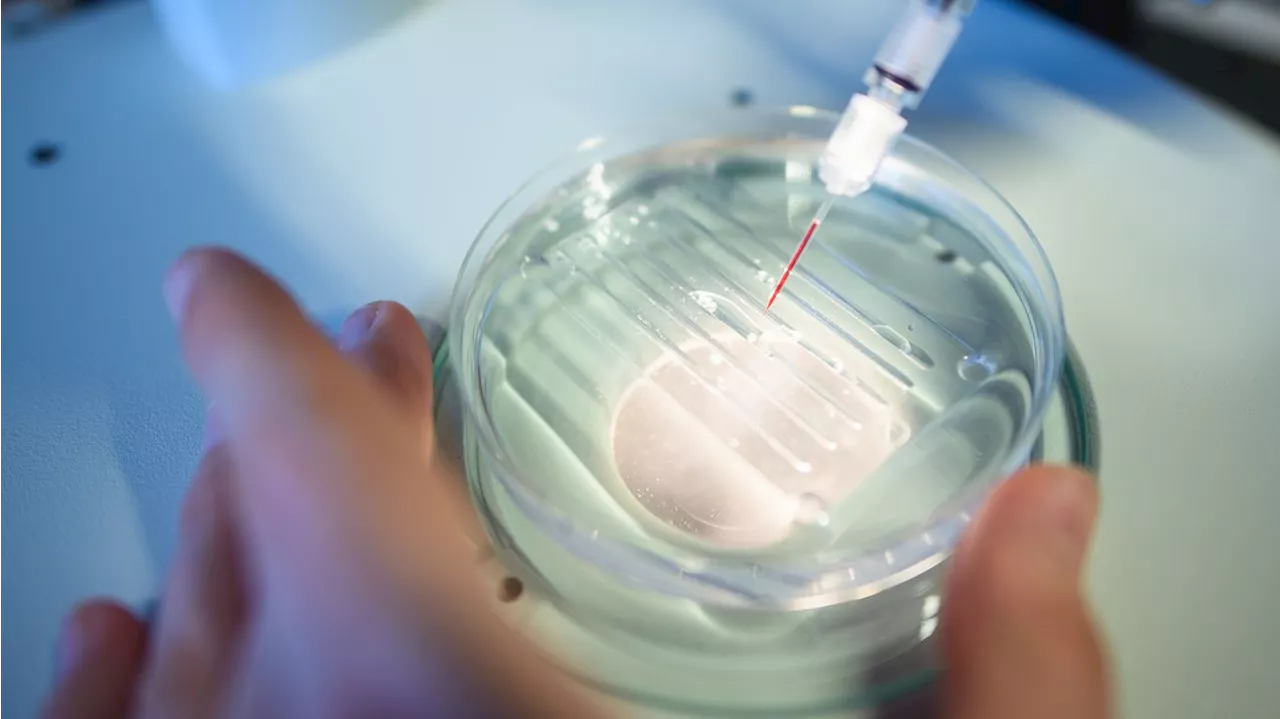 FDA panel weighs CRISPR-based sickle cell therapyThe FDA must decide whether to approve the therapy by Dec. 8.
FDA panel weighs CRISPR-based sickle cell therapyThe FDA must decide whether to approve the therapy by Dec. 8.
Lire la suite »
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Lire la suite »
 FDA considers 1st CRISPR gene editing treatment that may cure sickle cell diseaseUp until now, the only real treatment has been a stem cell or bone marrow transplant. The new exa-cel treatment under FDA consideration can use the patient's own stem cells.
FDA considers 1st CRISPR gene editing treatment that may cure sickle cell diseaseUp until now, the only real treatment has been a stem cell or bone marrow transplant. The new exa-cel treatment under FDA consideration can use the patient's own stem cells.
Lire la suite »
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Lire la suite »
