The authorization could be official by the end of February.
A covid-19 vaccine for the youngest American children may be here sooner than expected. This week, Pfizer will reportedly
apply for an emergency use authorization of their low-dose vaccine for children under five. Should the Food and Drug Administration grant the authorization, the vaccine could be available as a two-dose series by the end of the month. But there are still questions about whether the current clinical data will be enough to convince the FDA, and it’s likely that a third dose would be recommended down the line, regardless.
Monday evening. It’s expected to call for the vaccine to be authorized as a two-shot schedule for children between six months and five years old. These shots would contain one-tenth the dose as the standard adult dose.last December by the company. They reported that children between the ages of two to five did not develop a robust-enough immune response to the two-dose vaccine when compared to younger individuals between the ages of 16 to 25.
At the time, Pfizer announced that it would extend the trial further by adding a third dose to the schedule for children 6 months to under 5 years of age. If the results from this trial went well, they then planned to submit for the EUA sometime in the “first half of 2022.” The third-dose trial is still ongoing. But it now appears that Pfizer will argue for the vaccine to be given as a two-dose series initially, with the expectation that the subsequent data will validate the added benefit of a third dose. It’s a decision that’s reportedly being encouraged by the Biden administration, in hopes of speeding along the rollout of the vaccine to younger children.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
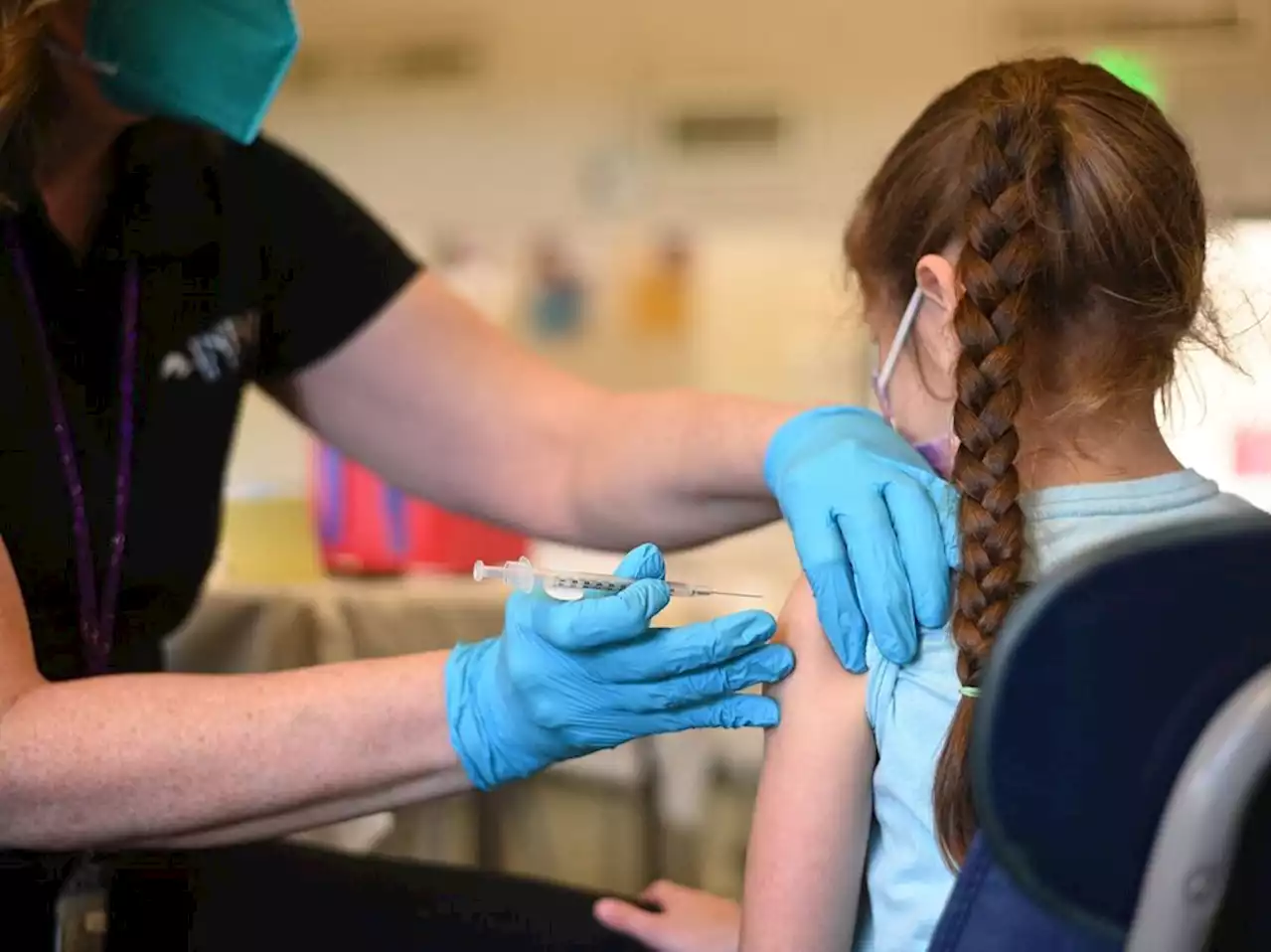 COVID-19 vaccine for young kids could be ready this monthThe last age group of the population unable to get a COVID-19 vaccine may soon be able to do so - and much earlier than anticipated:
COVID-19 vaccine for young kids could be ready this monthThe last age group of the population unable to get a COVID-19 vaccine may soon be able to do so - and much earlier than anticipated:
Lire la suite »
 Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Lire la suite »
 Moderna COVID-19 vaccine gets full FDA approvalThe Moderna Covid-19 vaccine received full approval from the U.S. Food and Drug administration Monday, 11 months after the shot was given an emergency use authorization.
Moderna COVID-19 vaccine gets full FDA approvalThe Moderna Covid-19 vaccine received full approval from the U.S. Food and Drug administration Monday, 11 months after the shot was given an emergency use authorization.
Lire la suite »
 Moderna's COVID-19 vaccine granted full FDA approval, company saysModerna says the Food and Drug Administration has issued full approval for its COVID-19 vaccine for use in people aged 18 and older.
Moderna's COVID-19 vaccine granted full FDA approval, company saysModerna says the Food and Drug Administration has issued full approval for its COVID-19 vaccine for use in people aged 18 and older.
Lire la suite »
 FDA Gives Moderna Full Approval for Its COVID-19 VaccineModerna says U.S. health regulators have given full approval to its COVID-19 vaccine after reviewing additional data on its safety and effectiveness
FDA Gives Moderna Full Approval for Its COVID-19 VaccineModerna says U.S. health regulators have given full approval to its COVID-19 vaccine after reviewing additional data on its safety and effectiveness
Lire la suite »
