“Who cares if the (Food and Drug Administration) says don’t make your own formula,” tweeted Liz Joy.
challenging federal rules and countless doctors who say homemade concoctions can harm infants.
“Who cares if the says don’t make your own formula – They aren’t doing anything to fix the formula shortage! And – our babies need quality formulas,” tweeted Liz Joy, the sole Republican running in the Capital District race. The FDA claims homemade formula can be easily contaminated and not include the critical nutrients growing infants need, both of which can be life-threatening.
Joy, a 53-year-old mother and grandmother, said she’s never had to make her own formula but sympathizes with the droves of parents who can’t breastfeed or find products such as Enfamil or Similac on store shelves. “Mothers have to feed their babies, and they don’t have time to wait for our government a week from now to sit down and have a committee hearing on what they should do,” she told The Post.
France Dernières Nouvelles, France Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
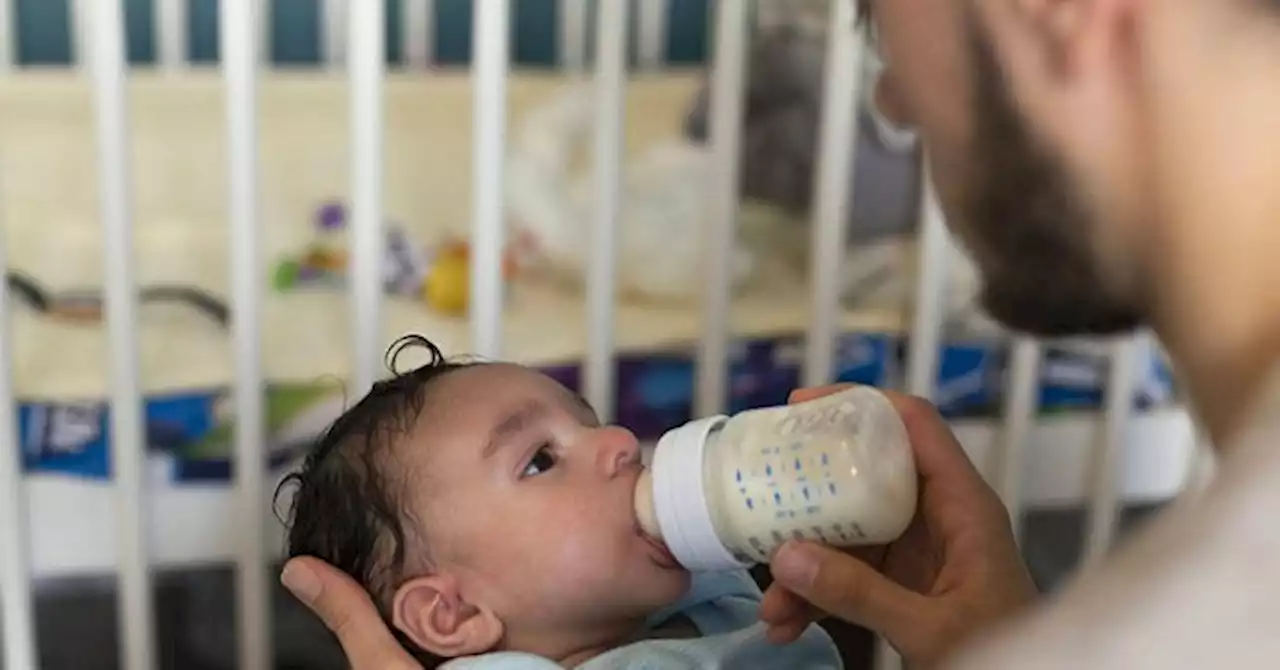 Texas Man Gives Free Baby Formula to Families Struggling to Find ItOne Texas restaurant owner is doing all he can to feed the babies in his community amid the baby formula shortage that has swept the nation.
Texas Man Gives Free Baby Formula to Families Struggling to Find ItOne Texas restaurant owner is doing all he can to feed the babies in his community amid the baby formula shortage that has swept the nation.
Lire la suite »
 Omar Leads Charge Against Baby Formula Monopolies Amid US ShortageDemocrats urge the FTC to probe \u0022any unfair or unsustainable practices, like deceptive marketing, price gouging, and stock buybacks, that may be weakening our nutritional formula supply.\u0022
Omar Leads Charge Against Baby Formula Monopolies Amid US ShortageDemocrats urge the FTC to probe \u0022any unfair or unsustainable practices, like deceptive marketing, price gouging, and stock buybacks, that may be weakening our nutritional formula supply.\u0022
Lire la suite »
 Babies hospitalized as formula shortage grows more direThe baby formula shortage has left parents frantic with the lack of formula sending at least four babies to a children's hospital in South Carolina.
Babies hospitalized as formula shortage grows more direThe baby formula shortage has left parents frantic with the lack of formula sending at least four babies to a children's hospital in South Carolina.
Lire la suite »
 FDA chief grilled on baby formula crisis, delivers mixed messageOn Thursday, Robert Califf said Abbott’s Michigan formula plant could be up and running by next week, but skirted questions about whether the FDA should have addressed problems at the facility sooner.
FDA chief grilled on baby formula crisis, delivers mixed messageOn Thursday, Robert Califf said Abbott’s Michigan formula plant could be up and running by next week, but skirted questions about whether the FDA should have addressed problems at the facility sooner.
Lire la suite »
